Bismuth-based self-supporting electrocatalyst, preparation method thereof and application of bismuth-based self-supporting electrocatalyst in nitrogen reduction ammonia production
An electrocatalyst and self-supporting technology, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of high energy consumption, harsh conditions, and large pollution, and achieve large specific surface area and layer The effect of high spacing and efficient recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
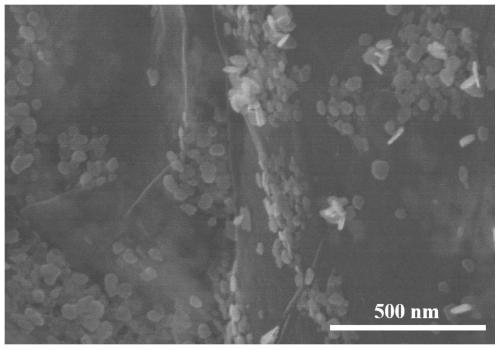
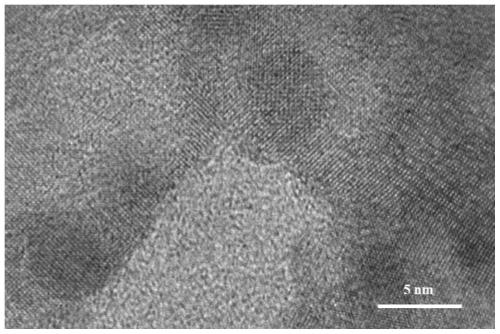
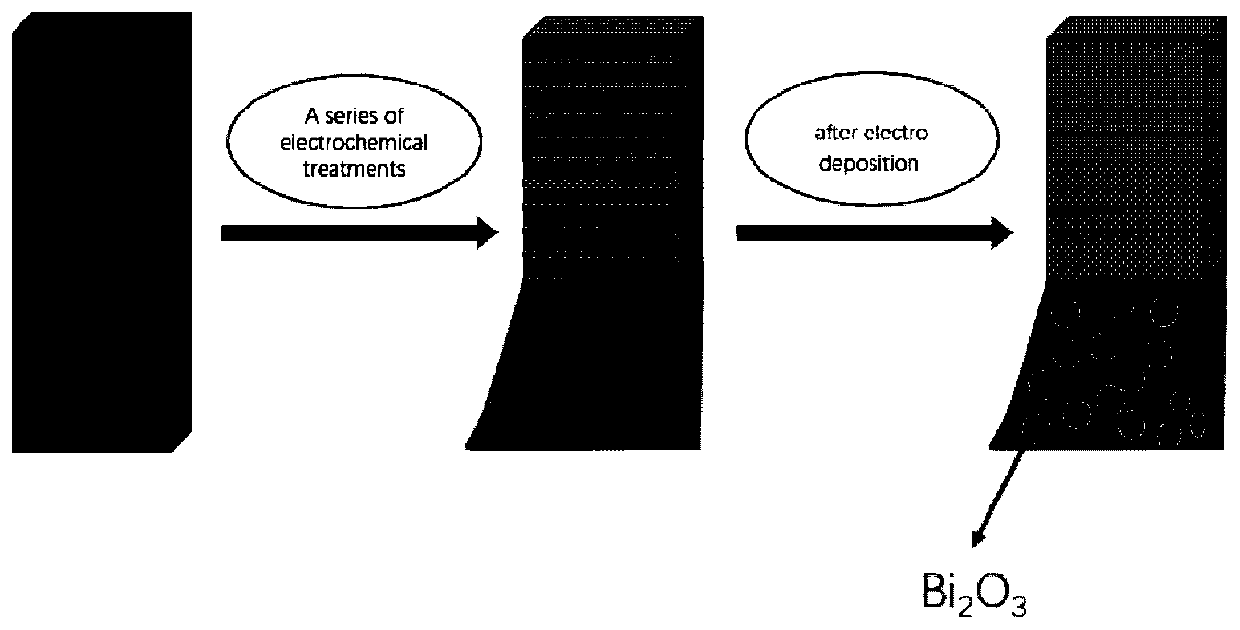
[0021] Embodiment 1 bismuth-based self-supporting electrocatalyst (Bi 2 o 3 @FEG)
[0022] (1) The preparation method is as follows:
[0023] 1) Preparation of FEG: carbon paper was used as working electrode, calomel electrode was used as reference electrode, and another piece of carbon paper was used as counter electrode. First, the carbon paper was charged at 20mV s by CV at a voltage range of 0.6 to 1.8V -1 Sweep 8 circles at the scanning speed, with 25mL0.5molL -1 K 2 CO 3 The solution was used as electrolyte, soaked in deionized water for 12h. Then, the carbon paper was scanned by i-t at a constant voltage of 1.8V for 9000s, and 25mL0.5mol L -1 KNO 3 The solution was used as electrolyte and washed several times with deionized water. Finally, the carbon paper was charged by CV at a voltage range of -1 to 0.9V at 50mV s -1 Sweep 50 circles at the scanning speed, with 25mL3 mol L -1 K 2 CO 3 The solution was used as an electrolyte, washed several times with wate...
Embodiment 2
[0031] Embodiment 2 bismuth-based self-supporting electrocatalyst (Bi 2 o 3 @FEG) Electrocatalytic Nitrogen Reduction for Ammonia Production
[0032] Experimental method: Under ambient conditions, the H-type electrolytic cell is used as the electrolytic cell, and 0.1M Na 2 SO 4 The solution is used as the electrolyte, the volume of the electrolyte is 70mL, the Ag / AgCl electrode is used as the reference electrode, the platinum sheet is used as the counter electrode, and the Bi 2 o 3 @FEG clips directly on the platinum electrode clip as the working electrode. Under nitrogen atmosphere, the electrocatalytic nitrogen reduction ammonia production experiment was carried out. After the experiment, the Bi 2 o 3 @FEG is washed several times with ultrapure water, and can be used directly after being washed several times with electrolyte solution before the next use.
[0033] The product was analyzed with an ammonia sensitive electrode. Such as Figure 4 and Figure 5 As shown, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com