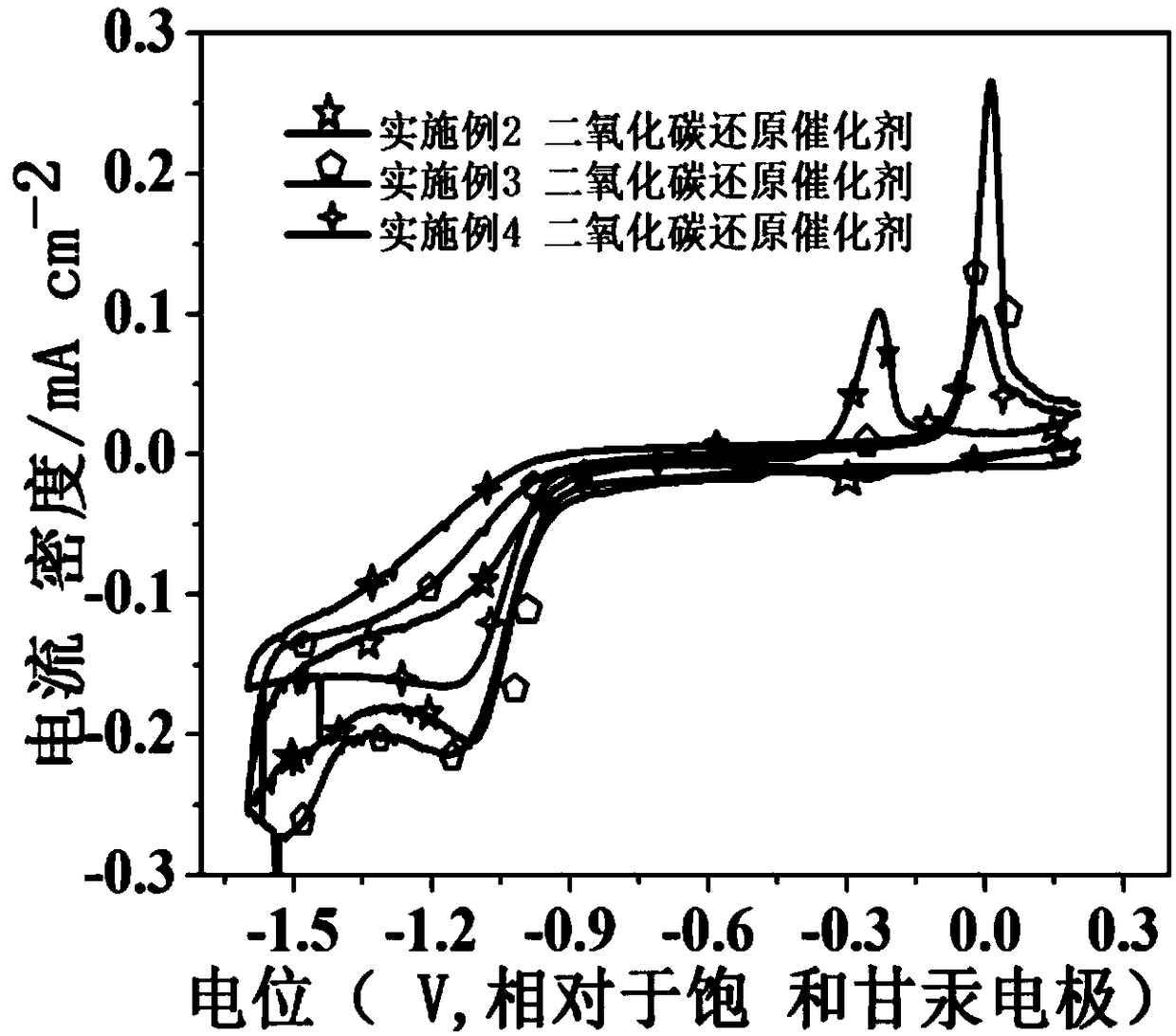
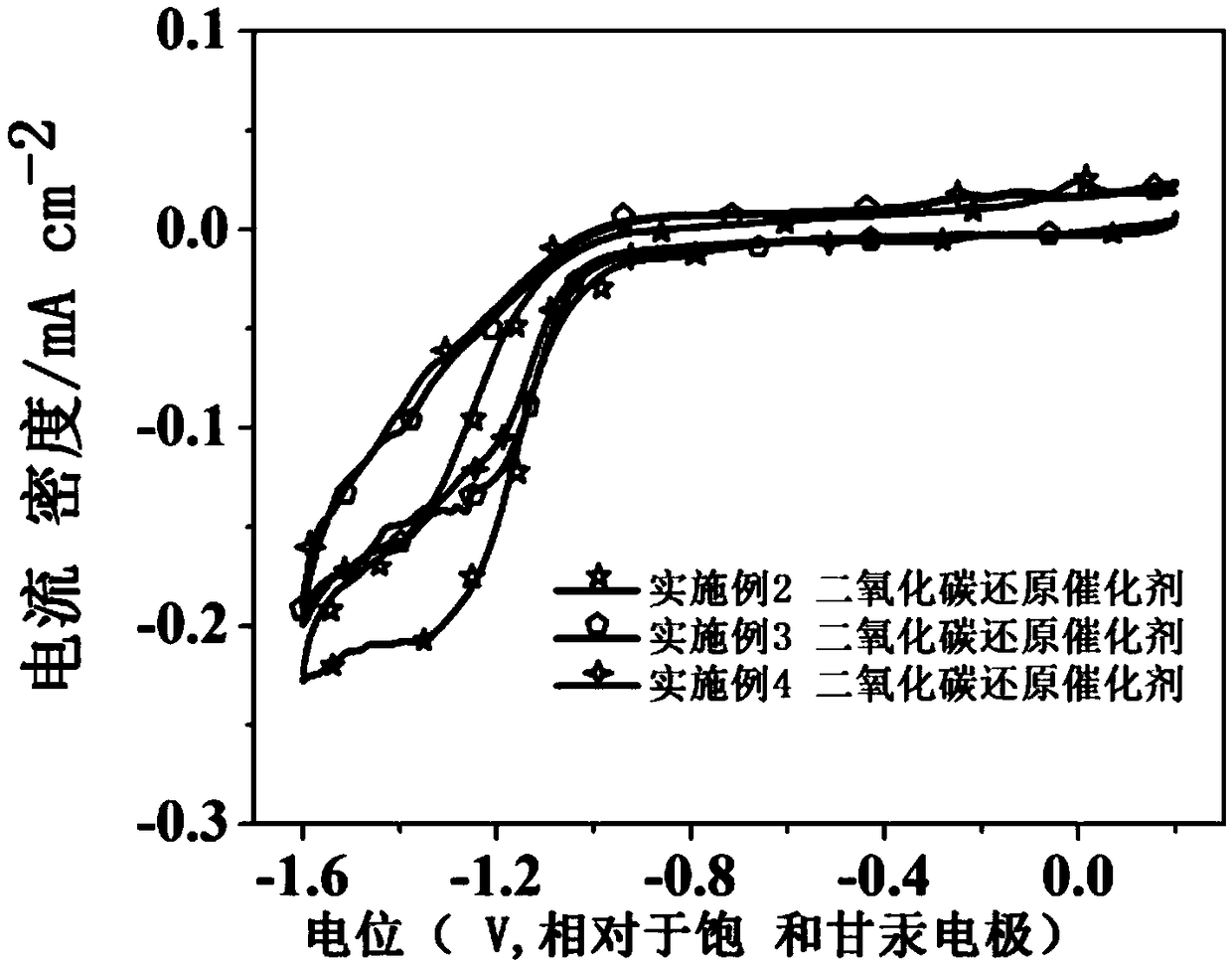
Carbon dioxide electrochemical reduction catalyst and preparation method thereof and catalyst loaded gas diffusion electrode
A technology of gas diffusion electrode and carbon dioxide, which is applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc. It can solve high overpotential, reduce formic acid yield, and accelerate hydrogen evolution Speed and other issues, to achieve the effect of inhibiting hydrogen evolution reaction, solving the problem of low reduction current and reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The present invention also provides a preparation method of a carbon dioxide electrochemical reduction catalyst, comprising the following steps:
[0032] Dissolve copper chloride or tin chloride, and nickel chloride in deionized water, stir, add ammonia water drop by drop to adjust the pH of the solution to 7.5-14, make the above metal ions co-precipitate, centrifuge, use deionized water and Wash with absolute ethanol until neutral, dry, and heat treat to obtain nickel-copper lamellar double metal oxide or nickel-tin lamellar double metal oxide. The heat treatment temperature is 250°C to 750°C, and the heat treatment time is 0.5 hours to 4 hours The most preferred reaction conditions are that the pH is 9, the heat treatment temperature is 350°C-450°C, and the heat treatment time is 3 hours. Preferably, the molar concentration ratio of the copper chloride to the nickel chloride is 1:9-9:1, and the molar concentration ratio of the tin chloride to the nickel chloride is 1:...
Embodiment 1
[0041] An electrochemical reduction catalyst for carbon dioxide, comprising a nickel-copper lamellar double metal oxide, the nickel-copper lamellar double metal oxide is synthesized by a co-precipitation method, and its preparation method is: take 2mmol copper chloride dihydrate, 8mmol Dissolve nickel chloride hexahydrate in 40mL deionized water in a beaker, dissolve 5mL of concentrated ammonia water in 45mL deionized water, add dropwise to the beaker until the pH value is 9, stir for 4h, centrifuge, use deionized water and no Wash with water and ethanol until neutral, and dry in a drying oven at 60°C to obtain a solid powder. The powder was ground and placed in a tube furnace and heated in air at 350 °C for 3 h to obtain a nickel-copper sheet-layer bimetallic oxide (the molar ratio of NiO / CuO was 8:2), which was used as a catalyst for the electrochemical reduction of carbon dioxide.
Embodiment 2
[0043] An electrochemical reduction catalyst for carbon dioxide, comprising a nickel-copper lamellar double metal oxide, the nickel-copper lamellar double metal oxide is synthesized by a co-precipitation method, and its preparation method is: take 2mmol copper chloride dihydrate, 8mmol Dissolve nickel chloride hexahydrate in 40mL deionized water in a beaker, dissolve 5mL of concentrated ammonia water in 45mL deionized water, add dropwise to the beaker until the pH value is 9, stir for 4h, centrifuge, use deionized water and no Wash with water and ethanol until neutral, and dry in a drying oven at 60°C to obtain a solid powder. The powder was ground and placed in a tube furnace and heated in air at 450 °C for 3 h to obtain a nickel-copper sheet-layer bimetallic oxide (the molar ratio of NiO / CuO was 8:2), which was used as a catalyst for the electrochemical reduction of carbon dioxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com