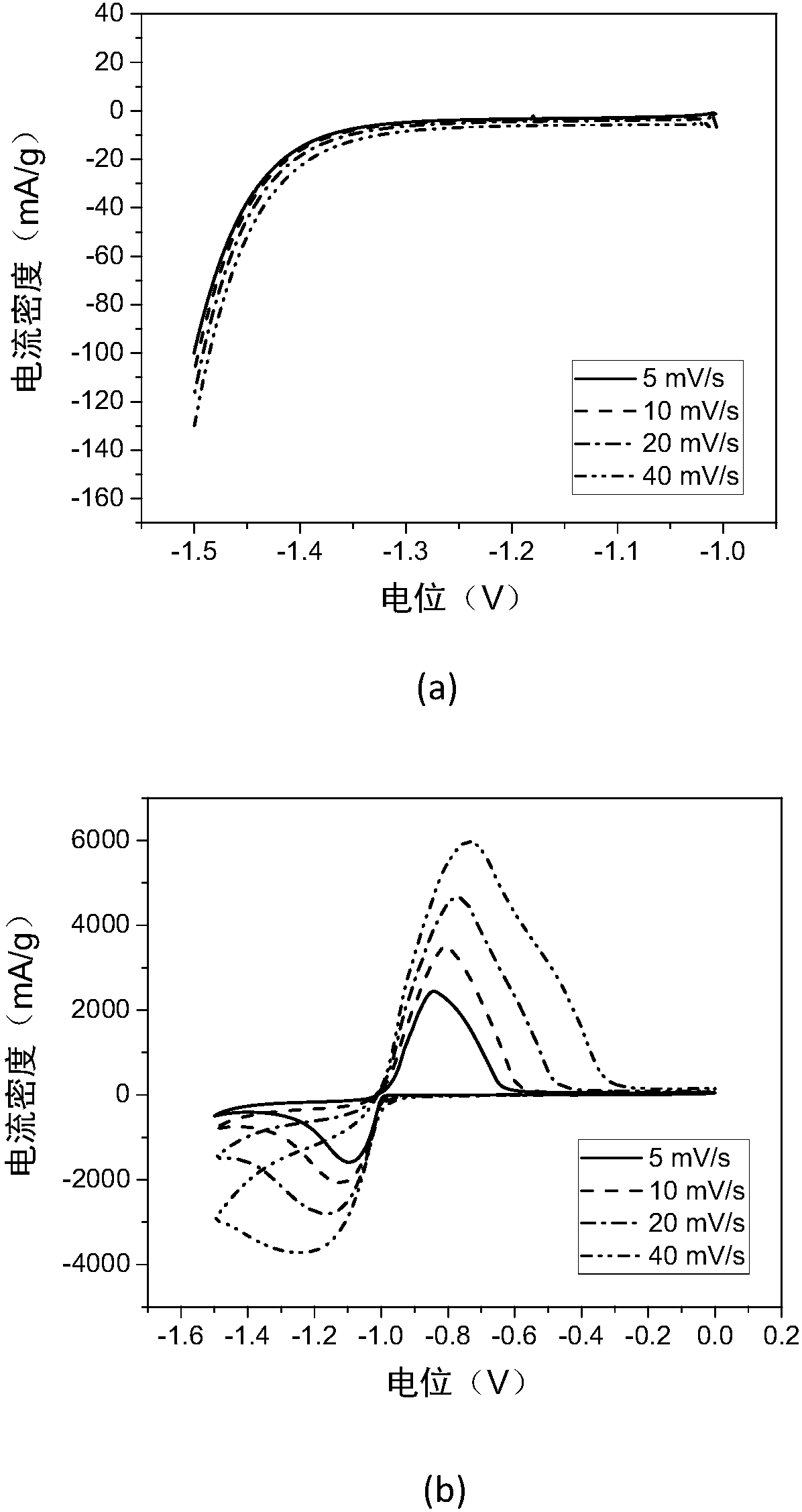
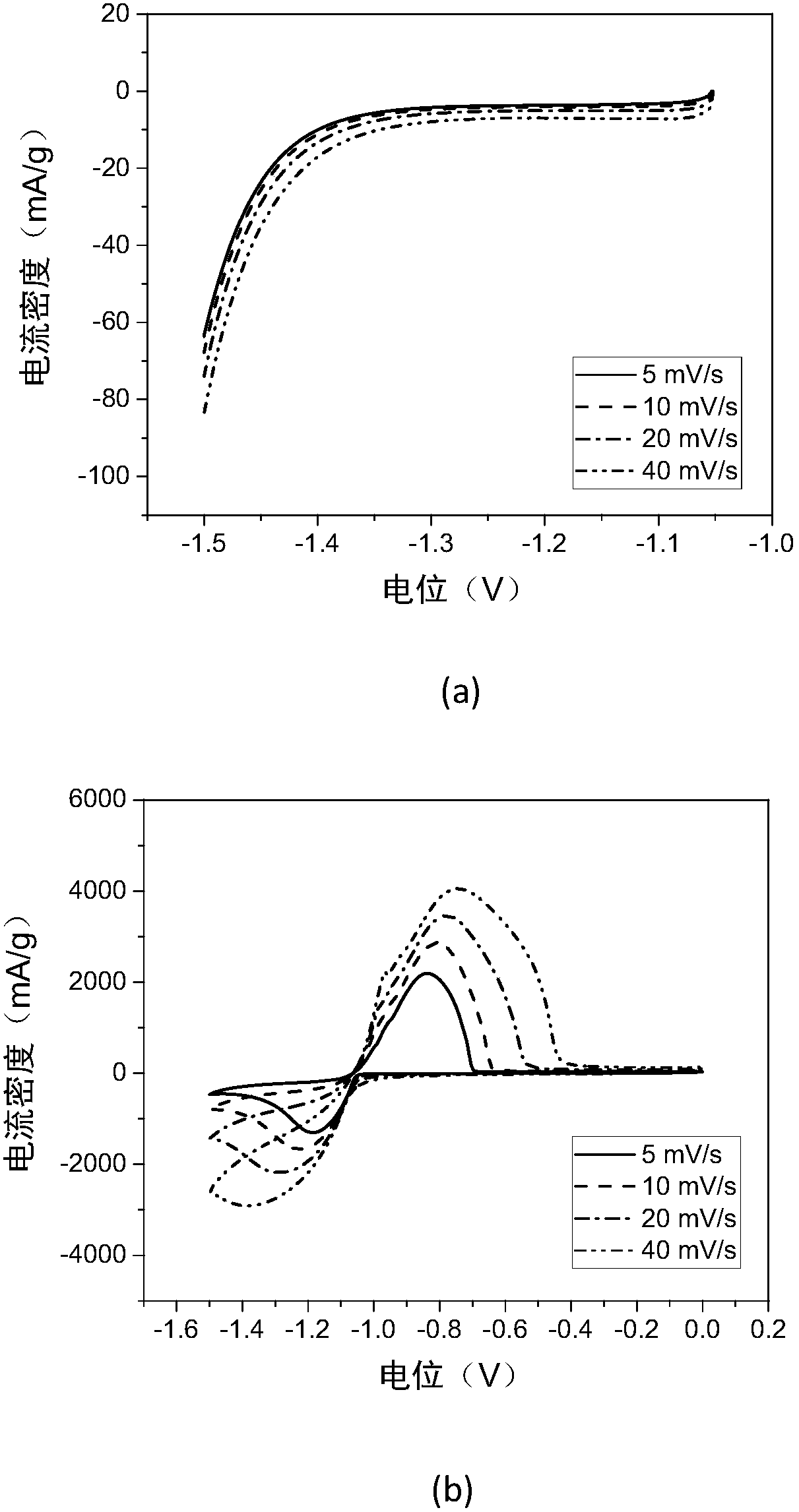
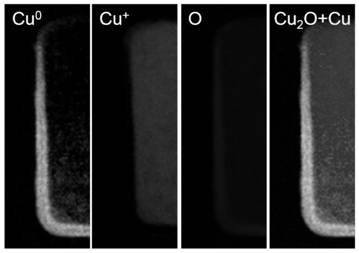
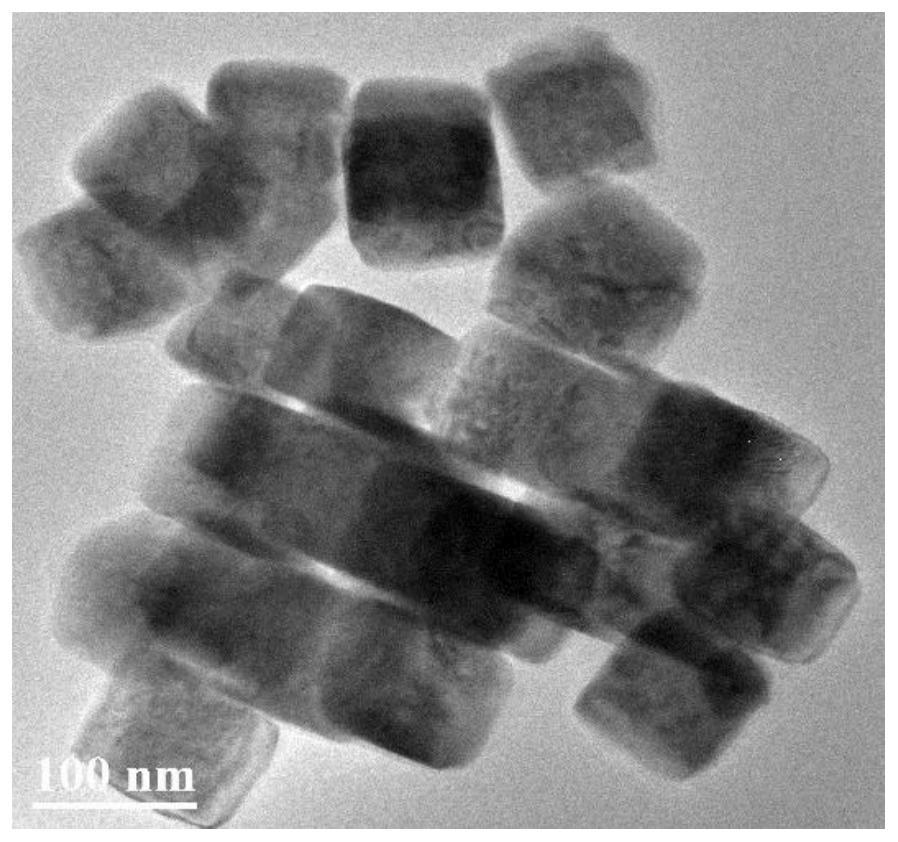
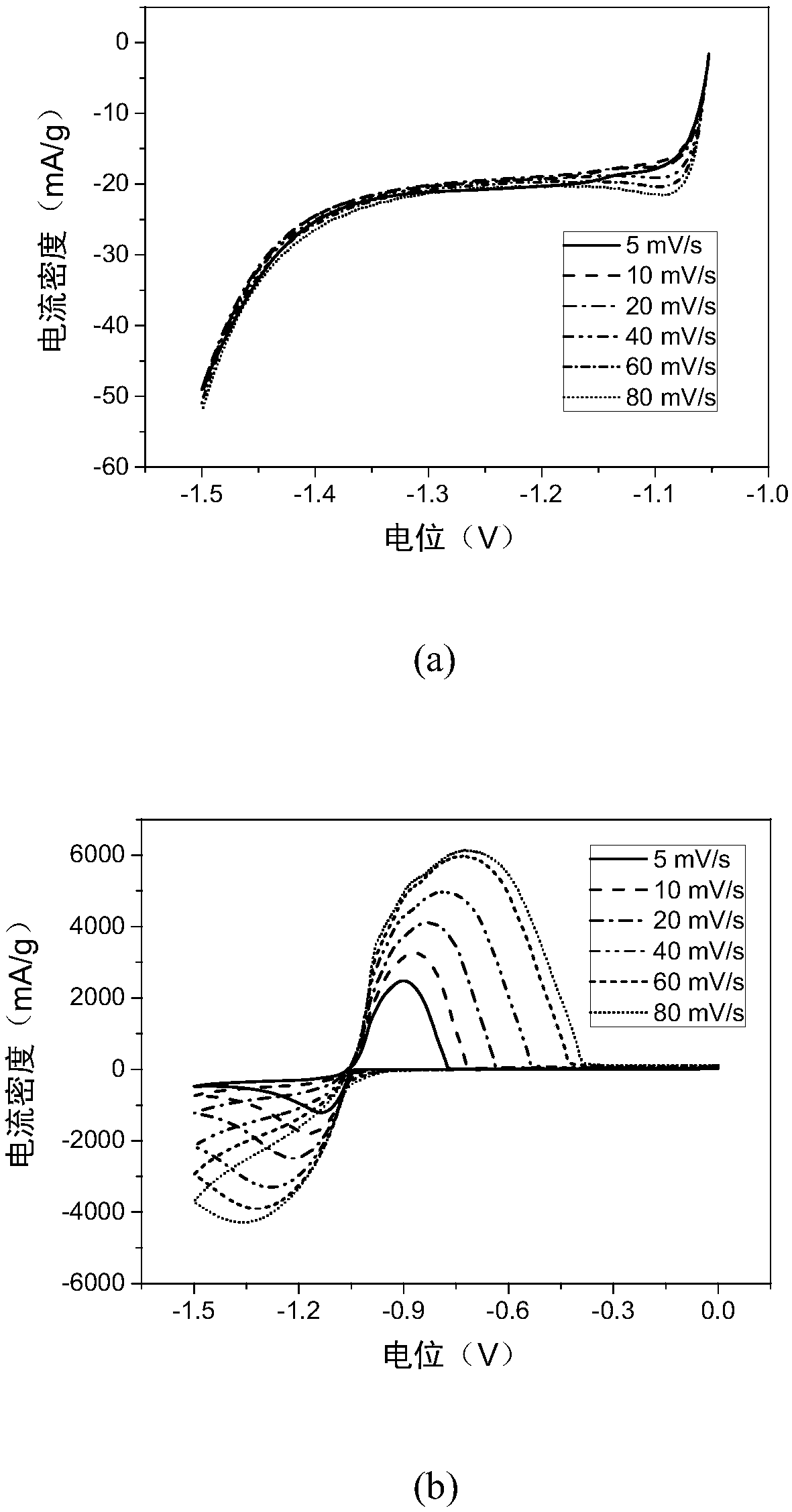
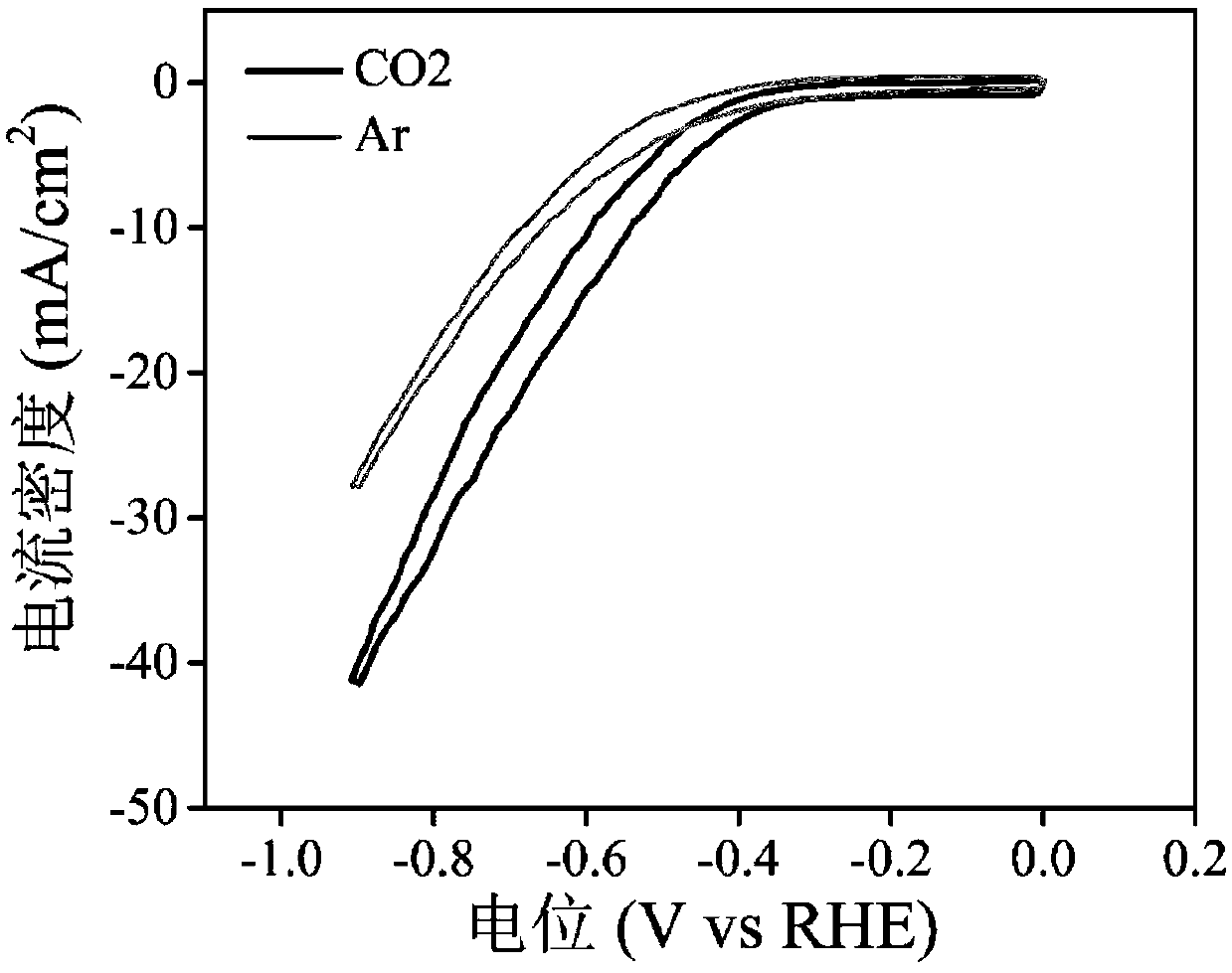
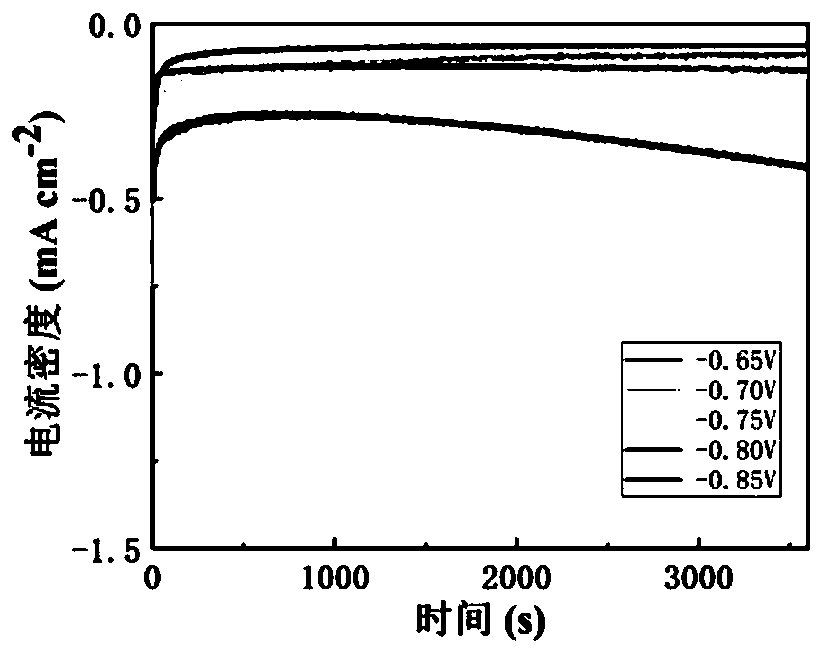
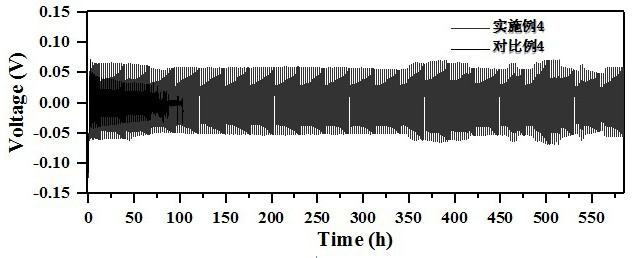
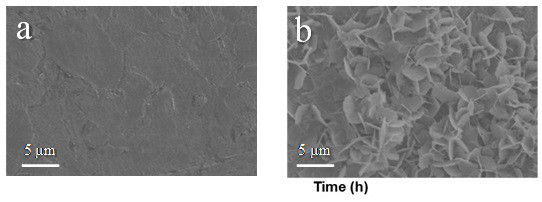
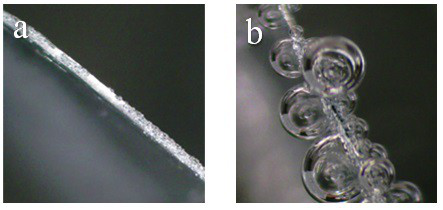
Patents
Literature
102results about How to "Inhibition of hydrogen evolution reaction" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
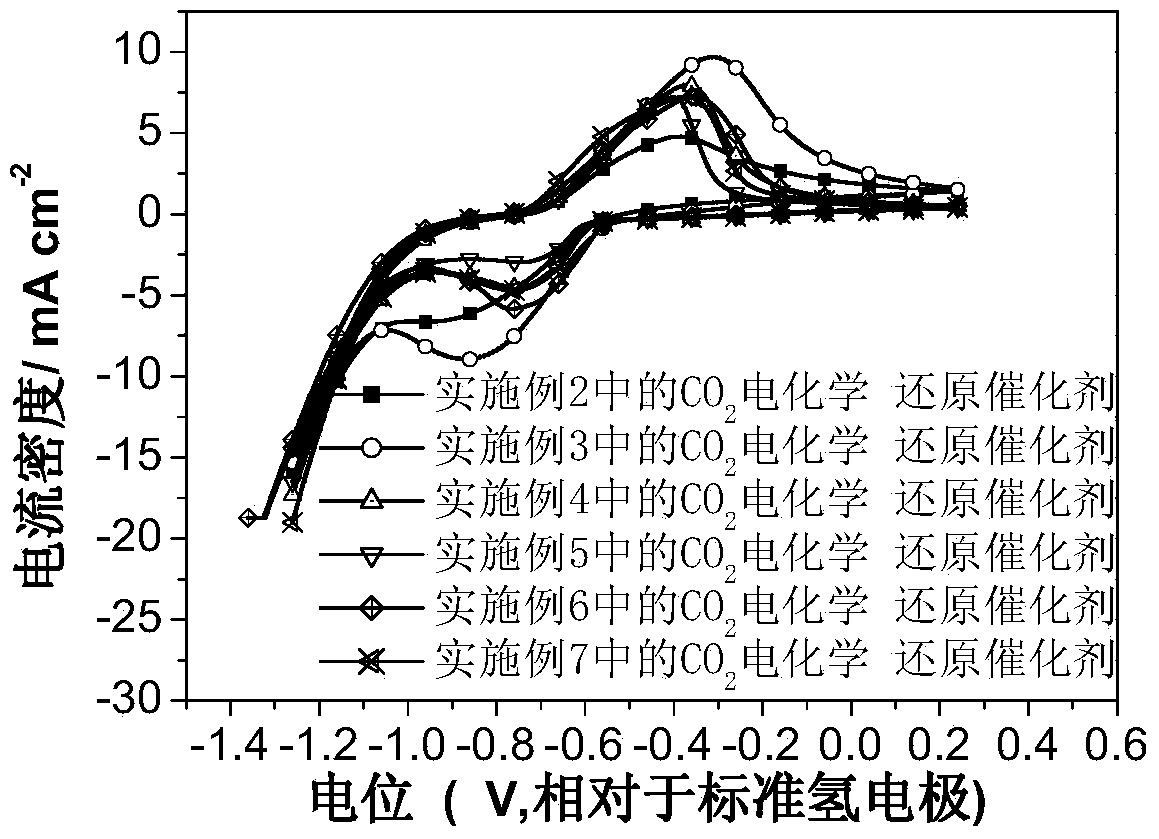
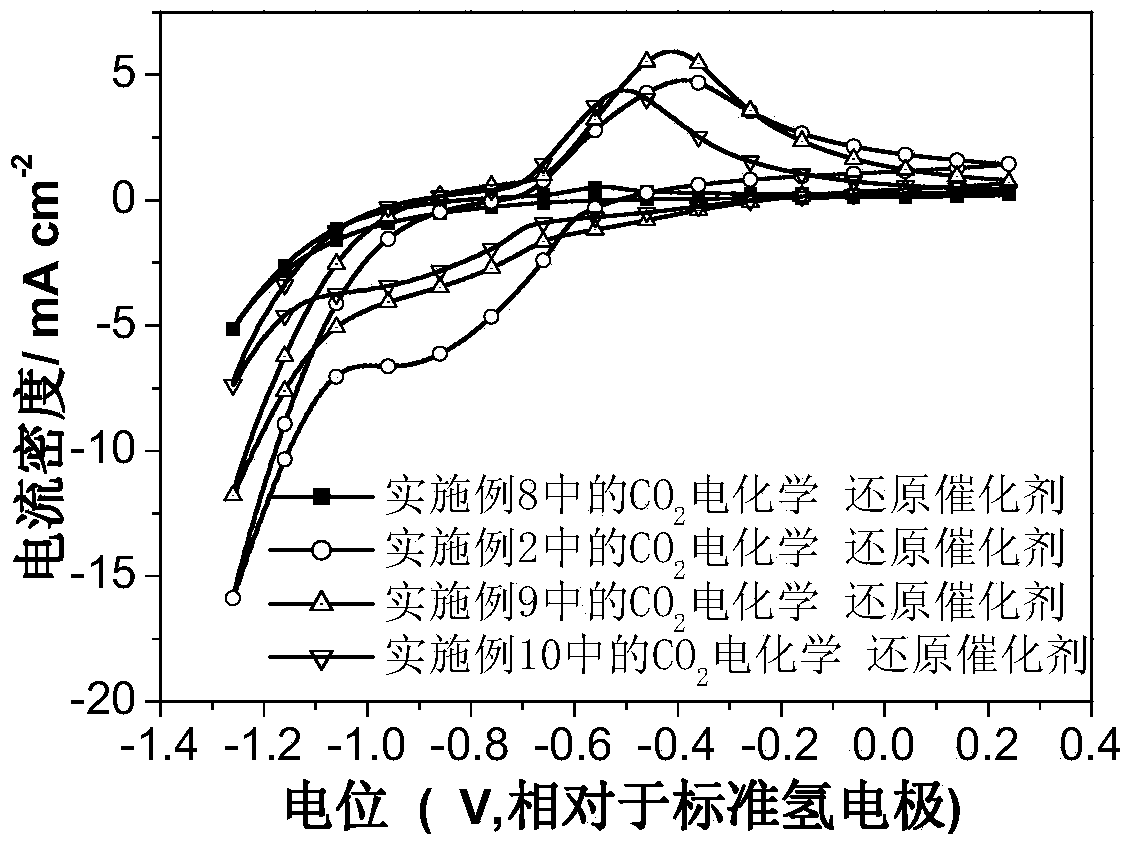
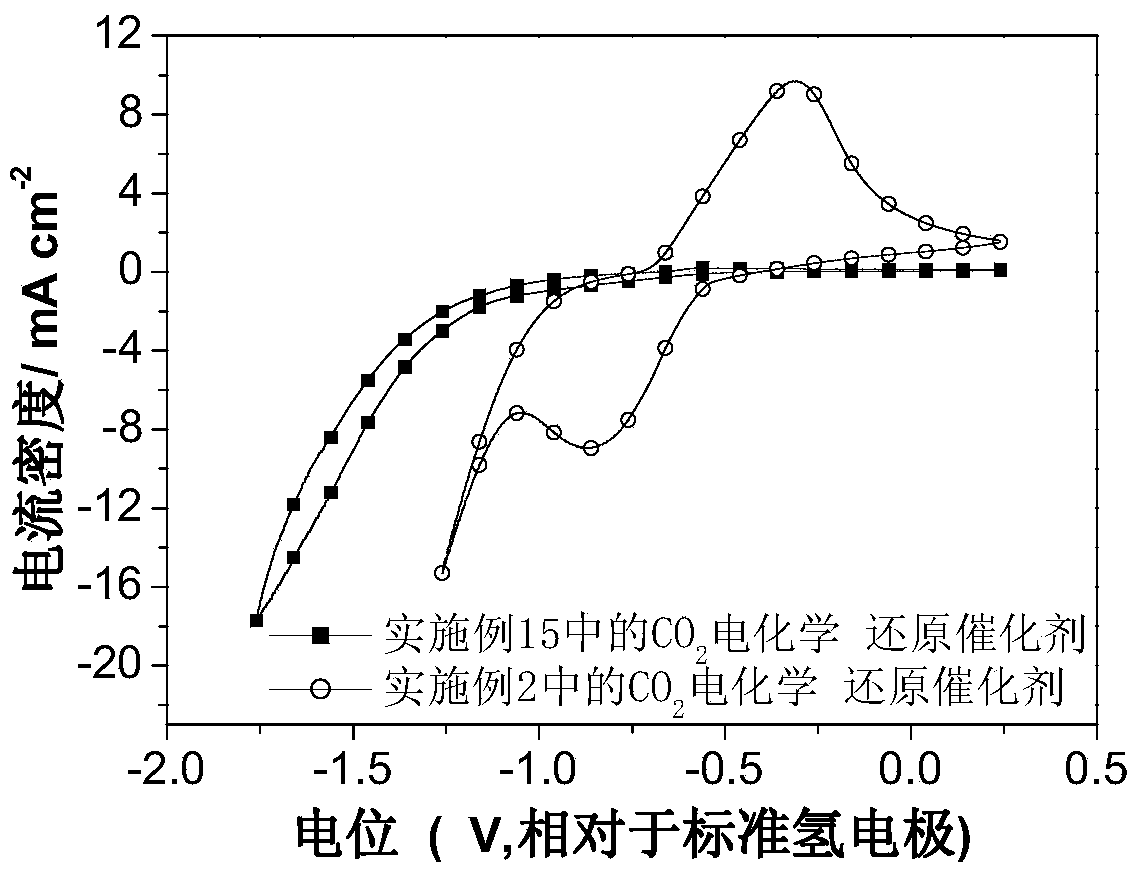
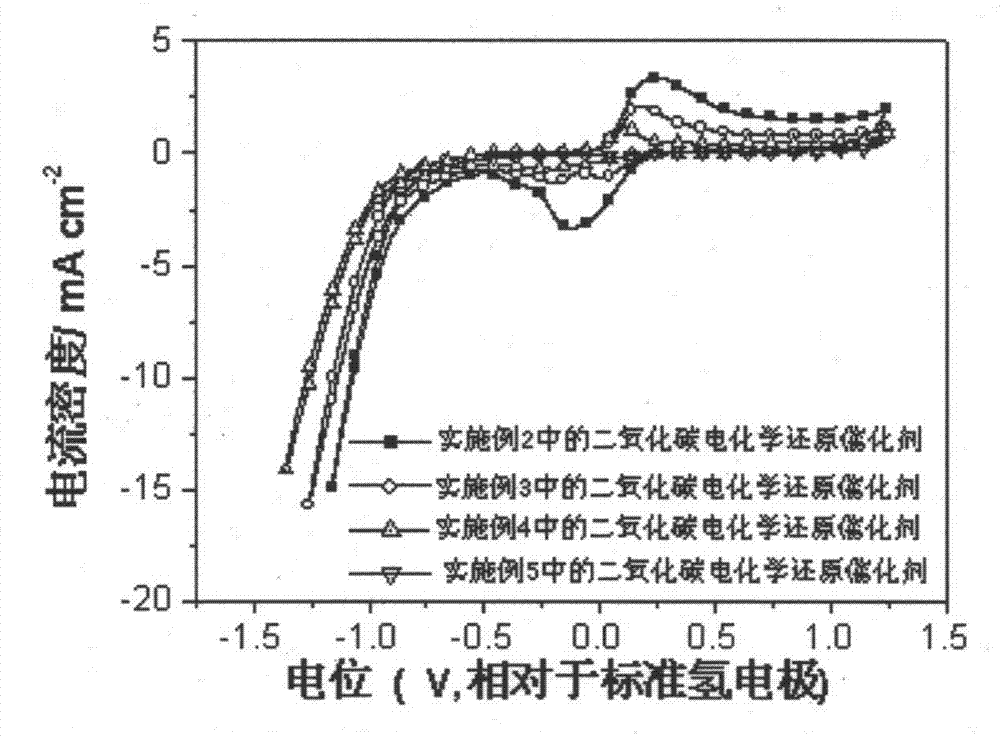
Carbon dioxide electrochemical reduction catalyst as well as preparation method and application thereof
InactiveCN103715436AIncrease the number of active sitesThe synthesis method is simpleMaterial nanotechnologyCell electrodesTin dioxideTrisodium citrate
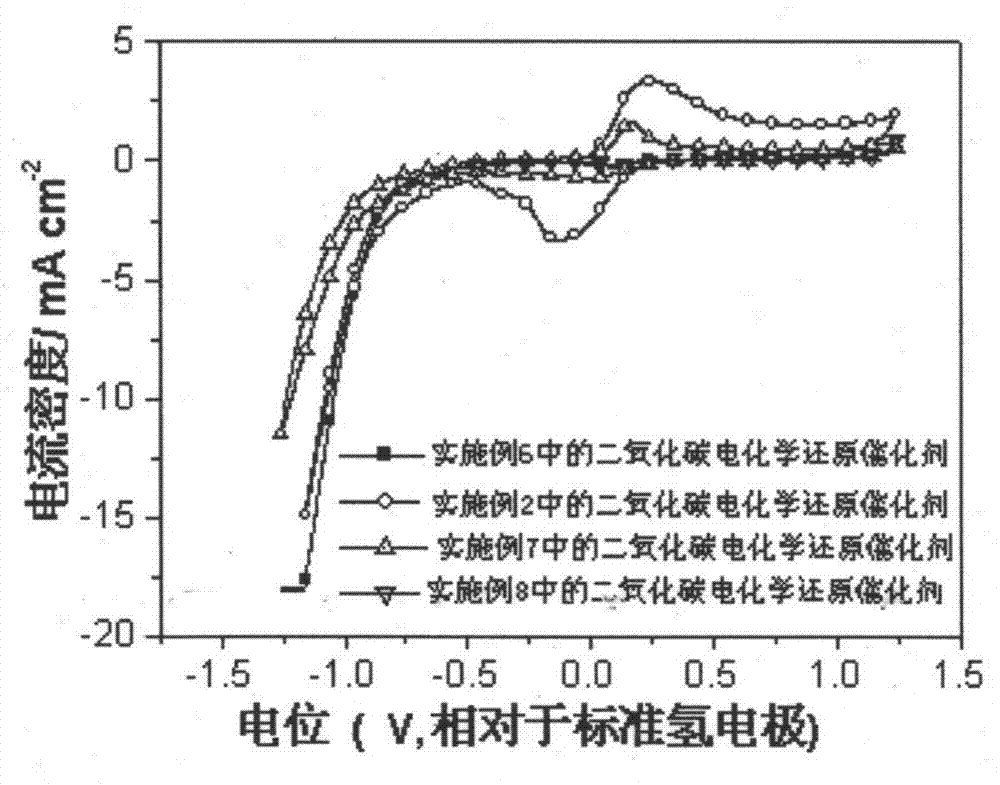
The invention relates to a carbon dioxide electrochemical reduction catalyst as well as a preparation method and an application thereof. The carbon dioxide electrochemical reduction catalyst comprises tin dioxide nanoflower, wherein the tin dioxide nanoflower is synthesized through hydrothermal reaction and comprises the synthesis raw materials including 0.1-0.5M of tin dioxide, 1-5M of trisodium citrate mixed solution and 0.1-0.5M of sodium hydroxide. The carbon dioxide electrochemical reduction catalyst is applied to a carbon dioxide electrochemical reduction catalyst gas diffusion electrode. The carbon dioxide electrochemical reduction catalyst has the advantages that the specific surface area of the catalyst is enlarged, the electrochemical reduction catalytic activity of the catalyst for carbon dioxide reduction is improved, the hydrogen evolution reaction is effectively inhibited, and the selectivity of formic acid in a product is improved.
Owner:DONGHUA UNIV +1
Carbon dioxide electrochemical-reduction catalyst, and preparation and application thereof
InactiveCN103566934AHigh specific surface areaIncrease contact areaMetal/metal-oxides/metal-hydroxide catalystsElectrodesM-AnisidineHydrogen
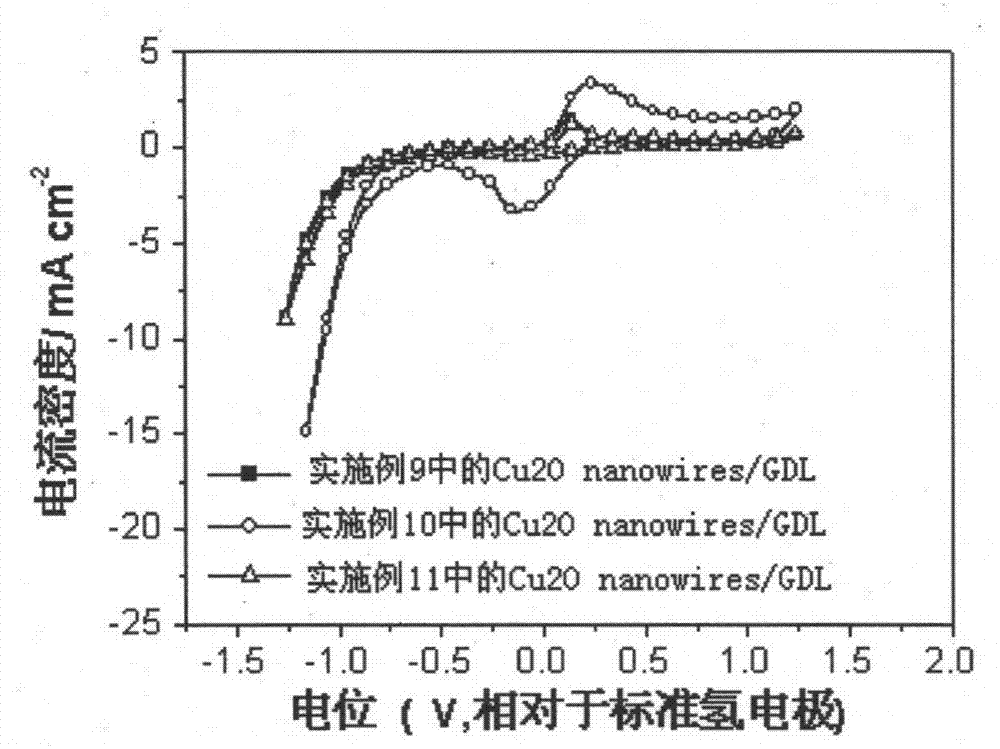
The invention provides a carbon dioxide electrochemical-reduction catalyst, and preparation and application thereof. The carbon dioxide electrochemical-reduction catalyst is characterized by comprising cuprous oxide nanowires, wherein the cuprous oxide nanowires are synthesized by hydrothermal reaction from 0.01-0.05M copper acetate and 0.01-0.05M anisidine at a volume ratio of 0.9:0.1 to 0.1:0.9. According to the carbon dioxide electrochemical-reduction catalyst, and the preparation and application thereof, the specific surface area of the catalyst is remarkably enlarged, the electrochemical reduction catalysis activity of the catalyst for the reduction of carbon dioxide is improved, and hydrogen evolution reaction is effectively inhibited.
Owner:DONGHUA UNIV
Method for preparing methane and ethylene through carbon dioxide electrochemical reduction
ActiveCN108588748AIncrease the catalytic activity of electrochemical reductionImprove Faraday efficiencyElectrolytic organic productionElectrodesFaraday efficiencyAuxiliary electrode
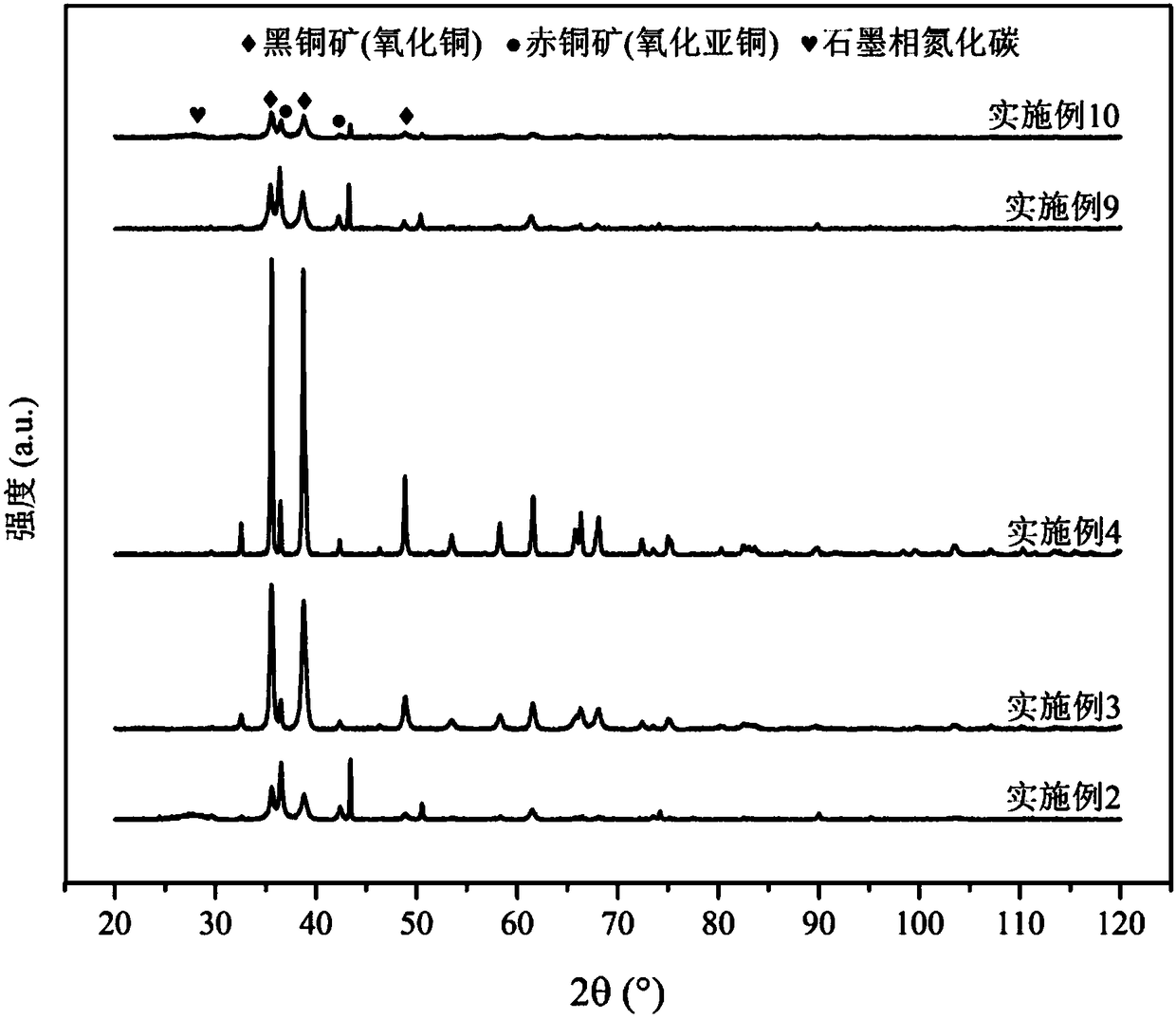
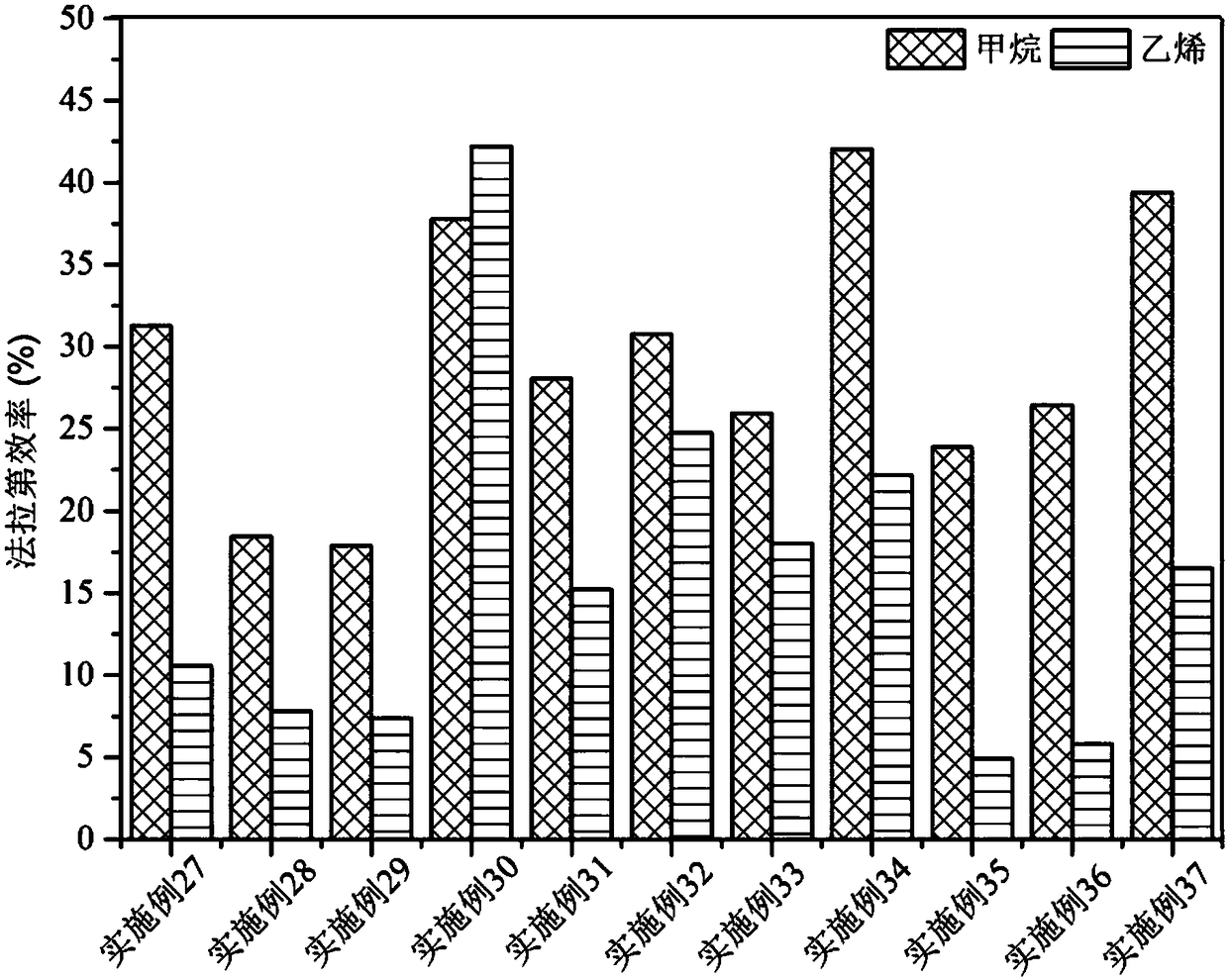
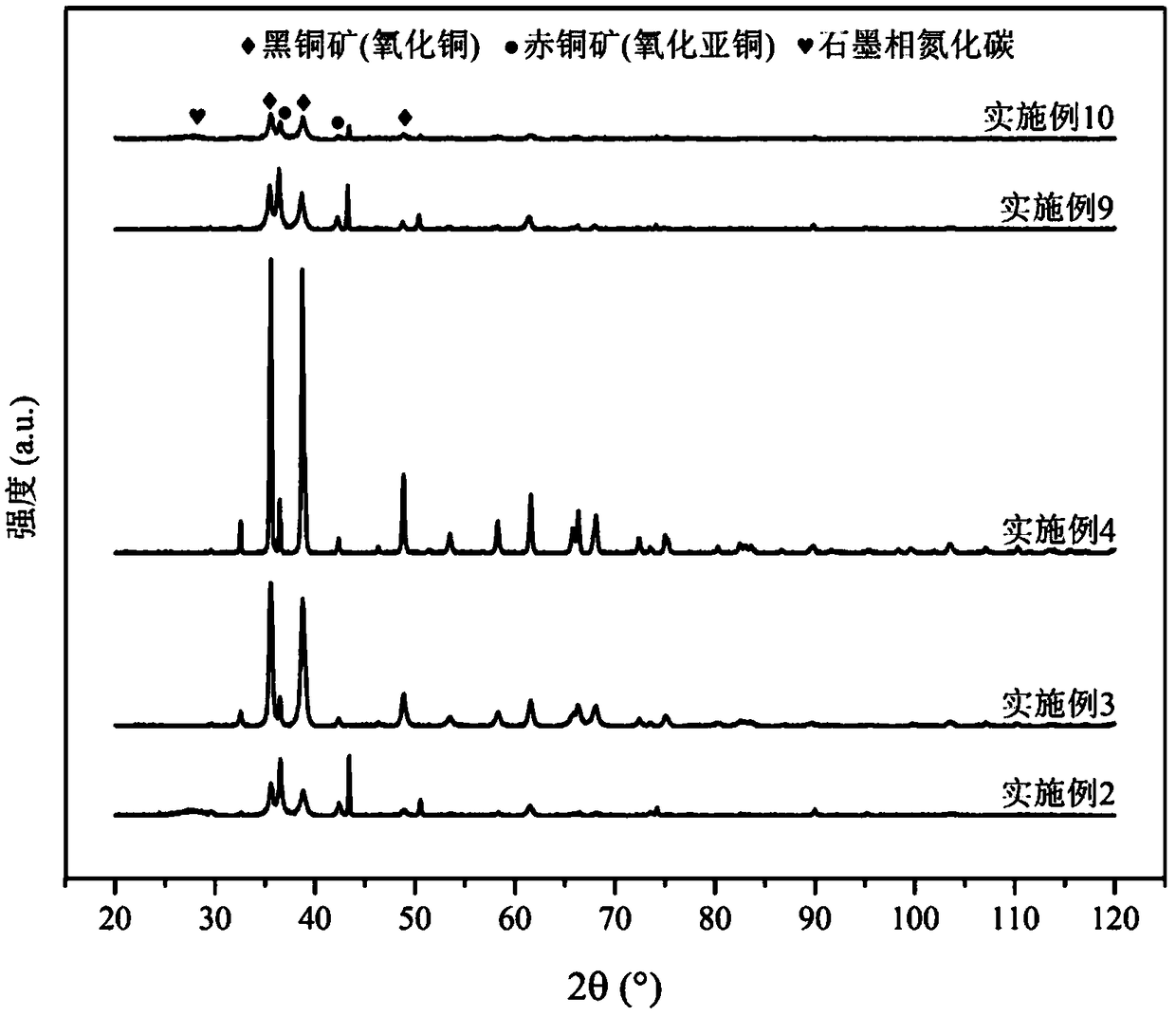
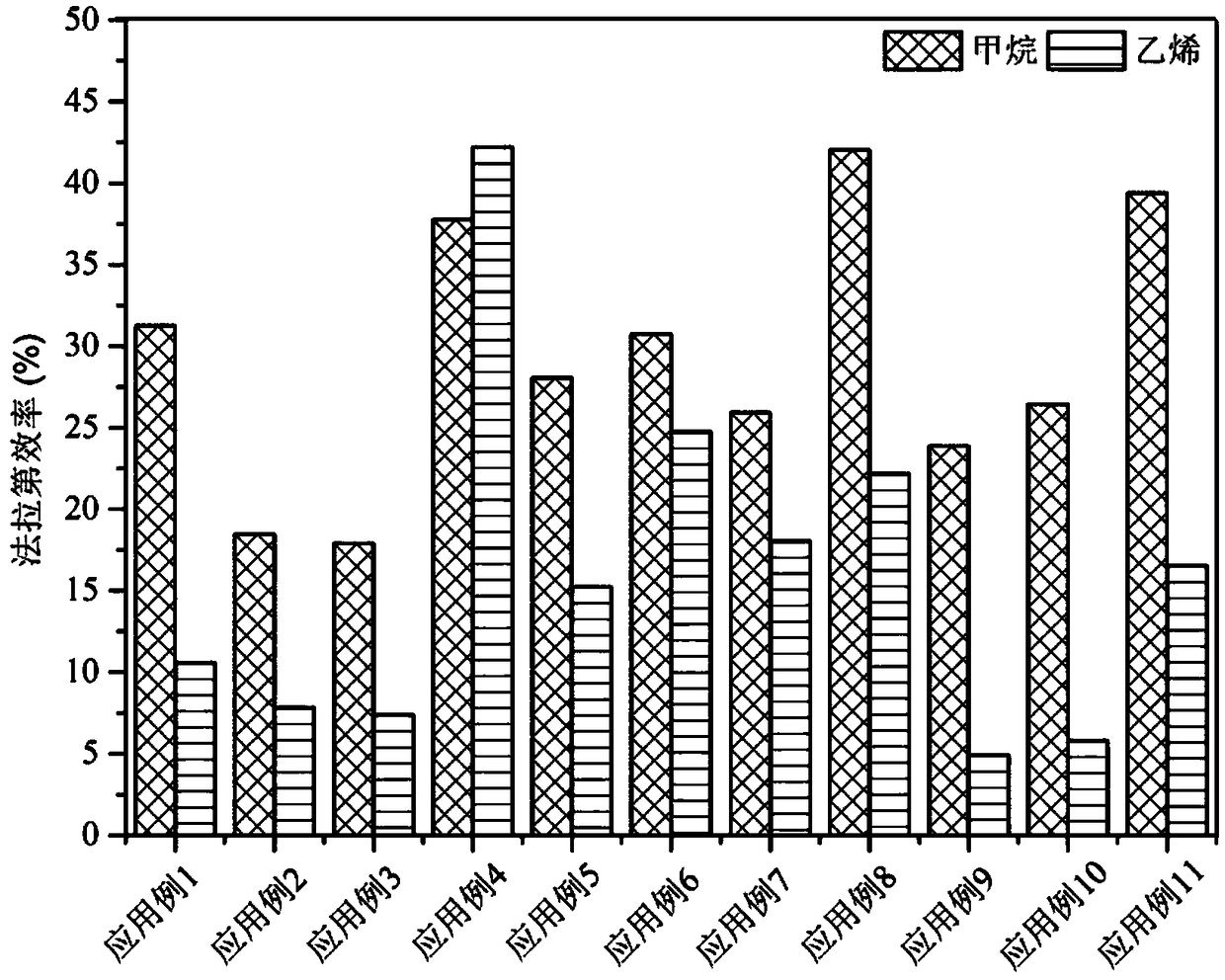
The invention relates to a method for preparing methane and ethylene through carbon dioxide electrochemical reduction. The method comprises the steps that an H-shaped dual-electrochemical-cell reactoris adopted, and is isolated into a cathode chamber and an anode chamber through a proton exchange membrane; before the reaction, carbon dioxide gas is led into the cathode chamber; a three-electrodesystem is adopted, a gas diffusion electrode serves as a work electrode, a platinum electrode serves as an auxiliary electrode, and a silver / silver chloride electrode serves as a reference electrode;the gas diffusion electrode comprises a gas diffusion electrode body and a carbon dioxide electrochemical reduction catalyst loaded gas diffusion electrode body; the carbon dioxide electrochemical reduction catalyst is graphite phase nitrogenated carbon supported nanometer copper oxide, and the nanometer copper oxide has tenorite and red copper ore. Through the method, the faradic efficiency of carbureted hydrogen of methane, ethylene and the like can be improved.
Owner:ZHEJIANG UNIV
Gas diffusion electrode, preparation method thereof and application thereof in electrochemical reduction of carbon dioxide
ActiveCN108823596AIncrease the catalytic activity of electrochemical reductionImprove Faraday efficiencyElectrolytic organic productionMetal/metal-oxides/metal-hydroxide catalystsFaraday efficiencyElectrochemical reduction of carbon dioxide
The invention relates to a gas diffusion electrode, a preparation method of the gas diffusion electrode, and the application of the gas diffusion electrode in electrochemical reduction of carbon dioxide. The gas diffusion electrode comprises a gas diffusion electrode body and carbon dioxide electrochemical reduction catalysts loaded on the gas diffusion electrode body. Nano copper oxide supportedon graphite phase carbon nitride serves as the carbon dioxide electrochemical reduction catalysts and has two crystal forms of tenorite and cuprit. The gas diffusion electrode can improve the Faradayefficiency of carbon dioxide electrochemical reduction products such as methane, ethylene and other hydrocarbons, and also can effectively inhibit hydrogen evolution reaction.
Owner:ZHEJIANG UNIV
Total-iron complexing flow cell with high open-circuit voltage
InactiveCN103700872AIncrease the open circuit voltageImprove efficiencyRegenerative fuel cellsAqueous electrolyte fuel cellsElectrode potentialHigh cell
The invention discloses a total-iron complexing flow cell with high open-circuit voltage. The total-iron complexing flow cell with high open-circuit voltage is characterized in that iron complexing water solutions are adopted for the positive electrode electrolyte and the negative electrode electrolyte. Iron ions and different types of complexing agents form iron complexing electrolyte, that is, ferrous / phenanthroline complexing electrolyte is adopted for the positive electrode, and ferric iron / triethanolamine complexing electrolyte is adopted for the negative electrode, so that the electrode potentials of ferrous / ferric iron electric pairs respectively move toward the positive direction and the negative direction. The total-iron complexing flow cell consisting of the two iron complexing electrolyte has relatively high cell open-circuit voltage. The iron complex also can inhibit the hydrogen evolution reaction, prevent cross pollution of the electrolyte solution comprising different types of ions, and improve the efficiency of the flow cell.
Owner:DALIAN UNIV OF TECH
Preparation method for composite negative electrode of lead-carbon battery, and composite negative electrode and application thereof
ActiveCN107845777AImprove performanceHigh bulk densityLead-acid accumulatorsLead-acid accumulator electrodesActivated carbonPorous carbon
The invention relates to a preparation method for a composite negative electrode of a lead-carbon battery, and the composite negative electrode and application thereof. The preparation method comprises the following steps: producing lead sulfate in porous carbon in situ so as to improve the bulk density of a carbon material and reduce the density difference between the carbon material and lead powder; and then uniformly mixing modified activated carbon, lead powder and additives by using a shearing process so as to prepare the high-performance composite negative electrode for the lead-carbon battery. The composite negative electrode for the lead-carbon battery prepared by using the method has higher charge-discharge reversibility, longer charge-discharge cycle life and higher charge acceptance than conventional lead-acid batteries, and is easy to realize large-scale production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Copper-based compound/copper nanoelectrode with interface synergistic effect and preparation and application of copper-based compound/copper nanoelectrode
ActiveCN112899709AGood choiceInhibition of hydrogen evolution reactionElectrolytic organic productionNanotechnologyNano catalystPtru catalyst
The invention belongs to the technical field of electrode materials, and particularly relates to a copper-based compound / copper nanoelectrode with an interface synergistic effect and a preparation method and application of the copper-based compound / copper nanoelectrode. The electrode comprises a conductive substrate and a copper-based compound / copper nano-catalyst loaded on the surface, the copper-based compound comprises but is not limited to cuprous oxide, copper nitride, copper oxide and other compounds. The invention also discloses a preparation method and application of the electrode. The electrode material with an interface regulation and control function is prepared for the first time, and the reaction energy barrier of carbon monoxide dimerization is reduced by utilizing the synergistic effect of monovalent copper / zero-valent copper, bivalent copper / zero-valent copper and bivalent copper / monovalent copper / zero-valent copper, so that the hydrogen evolution reaction can be well inhibited during electrochemical carbon dioxide reduction, and good selectivity is achieved on multi-carbon products such as ethylene, ethanol and isopropanol.
Owner:BEIJING UNIV OF CHEM TECH
Lead paste for negative electrode of lead-carbon battery, preparation method of lead paste, polar plate for negative electrode of lead-carbon battery, and lead-carbon battery
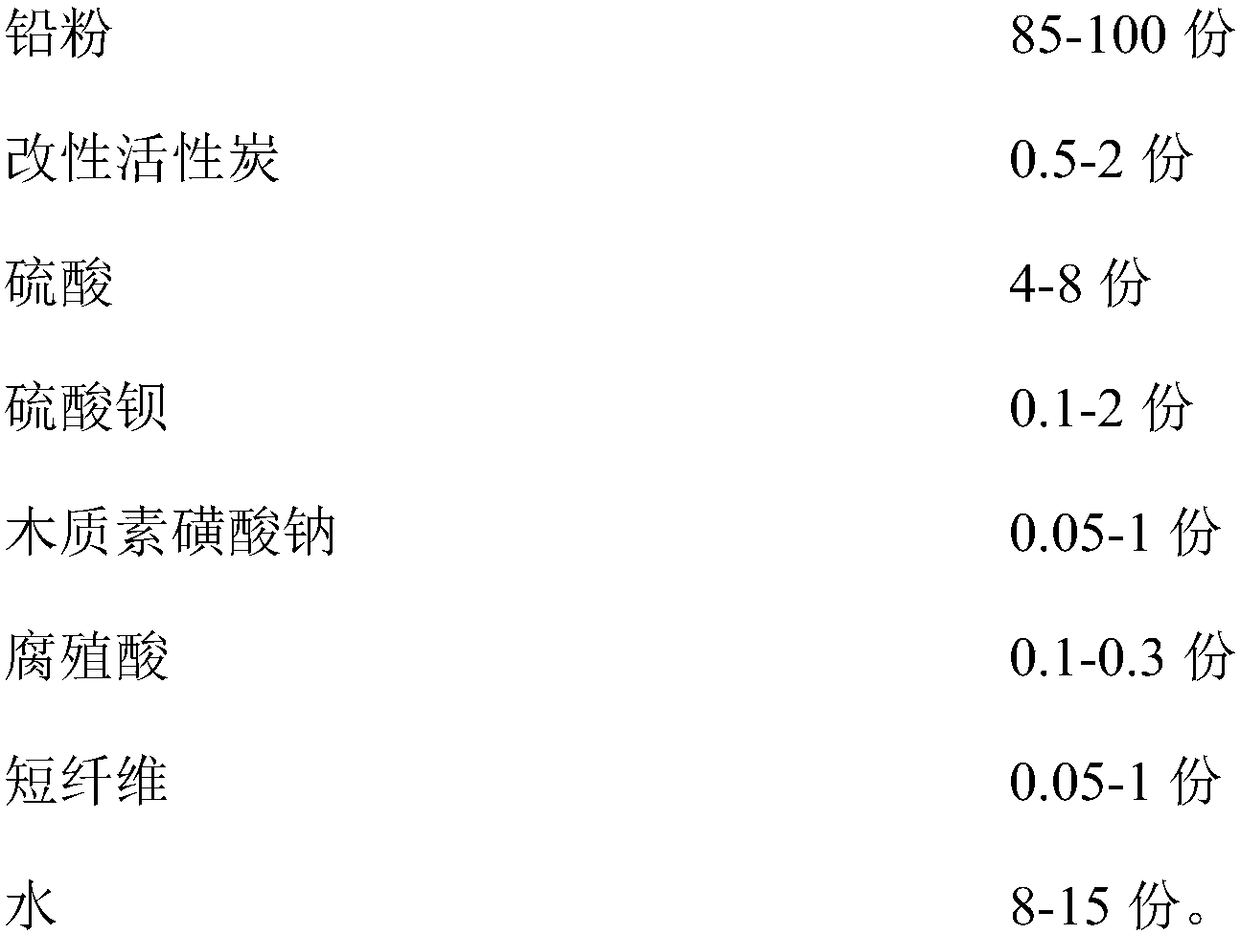
ActiveCN108493448AIncrease profitInhibition of hydrogen evolution reactionLead-acid accumulatorsCell electrodesFiberActivated carbon
The invention provides a lead paste for negative electrode of a lead-carbon battery, a preparation method of the lead paste, a polar plate for the negative electrode of the lead-carbon battery, and the lead-carbon battery. The lead paste for the negative electrode of the lead-carbon battery comprises the following components in parts by weight: 85-100 parts of lead powder, 0.5-2 parts of modifiedactivated carbon, 4-8 parts of sulfuric acid, 0.1-2 parts of barium sulfate, 0.05-1 part of sodium lignosulfonate, 0.1-0.3 part of humic acid, 0.05-1 part of short fibers, and 8-15 parts of water. Thepreparation method comprises the following steps: taking and mixing lead powder, modified activated carbon, sulfuric acid, barium sulfate, sodium lignosulfonate, humic acid, short fibers and water according to proportioning quantities to obtain the lead paste for the negative electrode of the lead-carbon battery. The polar plate for the negative electrode of the lead-carbon battery, provided by the invention, comprises the lead paste for the negative electrode of the lead-carbon battery, and the lead-carbon battery comprises the polar plate for the negative electrode of the lead-carbon battery. The lead paste for the negative electrode, provided by the invention, inhibits a hydrogen evolution reaction and improves the utilization rate of a carbon material in a negative electrode plate.
Owner:江苏德新管道科技有限公司
Lead-carbon battery composite negative electrode additive, preparation method and applications thereof
ActiveCN108123136ASimple preparation processEnergy savingLead-acid accumulator electrodesGraphite oxideHigh activity
The invention relates to a lead-carbon battery composite negative electrode additive and a preparation method thereof, wherein the lead-carbon battery composite negative electrode additive is formed by coating the surface of a reduced graphene oxide lamella with a metal element modified porous carbon material, and contains 0.1-50 wt% of reduced graphene oxide, 40-90 wt% of porous carbon, and 0.1-10 wt% of a metal element. According to the present invention, the lead-carbon battery composite negative electrode additive has high specific surface area and high electronic conductivity; and by doping the lead-carbon battery composite negative electrode additive into a lead-acid battery negative electrode, the lead-carbon battery negative electrode with characteristics of high activity, high charge-discharge reversibility and low hydrogen evolution can be obtained.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Nanometer tin/carbon composite material and preparation method thereof
InactiveCN108134091AAvoid bulky metallic tin situationsEvenly distributedMaterial nanotechnologyAlkaline accumulatorsCarbon compositesPotassium borohydride
The invention relates to a nanometer composite material, in particular to a nanometer tin / carbon composite material and a preparation method thereof. A nanometer tin and carbon compound is obtained bytaking various carbon materials as a substrate, tin nitrate, tin chloride, tin sulfate, tin acetate, tin citrate and the like as a tin source, water containing an organic complexing agent, ethylene glycol, propylene glycol or other mixture as a solvent and sodium borohydride, potassium borohydride, hydrazine hydrate and the like as a reducing agent and by an absorption-decomposition-reduction method. According to the method, a solution containing tin ions is absorbed to a surface of a carbon material, remaining solution is filtered, the tin oxide / tin and carbon compound is obtained after thermal treatment, and the nanometer tin / carbon composite material is finally obtained by reduction reaction. In the composite material obtained by the method, metal tin particles are uniformly distributed in surfaces of carbon particles in nanometer size, and a phenomenon that a large amount of tin can be agglomerated by a traditional tin reduction method is prevented.
Owner:CENT SOUTH UNIV
Electroreduction carbon dioxide catalytic material as well as preparation and application thereof
PendingCN111229195AChange the specific surface areaSuitable for industrial applicationsPhysical/chemical process catalystsElectrodesDoped graphenePtru catalyst
The invention discloses a preparation method and application of a carbon material. The carbon material is obtained through high-temperature carbonization synthesis, and synthesis raw materials comprise melamine and amino acid. The structure of the material is nitrogen-doped graphene, and the material is applied to a carbon dioxide electrochemical reduction catalyst gas diffusion electrode. According to the invention, the CO2 electroreduction activity of the catalyst is significantly improved, the catalytic stability is improved, the hydrogen evolution reaction is effectively inhibited, and theselectivity of the product CO is enhanced.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Reduction catalytic material, gas diffusion electrode and preparation method of gas diffusion electrode
ActiveCN108636402AIncrease the catalytic activity of electrochemical reductionGuaranteed contact areaCatalyst activation/preparationElectrolytic organic productionCarbonizationSurface binding
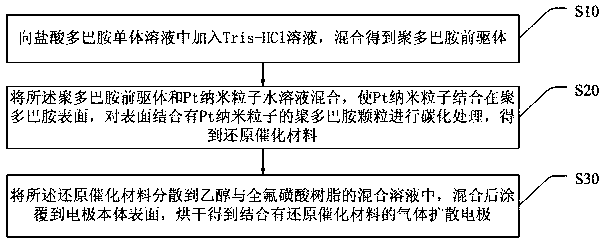
The invention discloses a reduction catalytic material, a gas diffusion electrode and a preparation method of the gas diffusion electrode. The reduction catalytic material comprises carbonized polydopamine particles and Pt nanoparticles bonded to the surfaces of the carbonized polydopamine particles. According to the invention, high-temperature carbonization treatment is carried out on the polydopamine particles with the Pt nanoparticles bonded to the surfaces, and then the specific surface area of the reduction catalytic material can be effectively improved, so that more active sites of the Pt nanoparticles are exposed, the electrochemical reduction catalytic activity of the reduction catalytic material to the reduction of carbon dioxide is increased, and the hydrogen evolution reaction is effectively inhibited. The surface of the gas diffusion electrode provided by the invention is combined with the reduction catalytic material. When the carbon dioxide is reduced by the electrode, the electrode can conduct the current and also can quickly discharge by-products such as H2, CH4 and the like generated by the reduction process out of the working electrode, so that the contact area ofthe CO2 and the reduction catalytic material is ensured, and the Faraday current efficiency is improved.
Owner:SHENZHEN UNIV
Copper wire nickel-plating electrolyte and preparation method thereof
The invention relates to the technical field of copper wire nickel plating and discloses copper wire nickel-plating electrolyte and a preparation method thereof. The copper wire nickel-plating electrolyte comprises the following materials: 300-350 g / L of nickel sulfate, 10-15 g / L of sodium hypophosphite, 32-40 g / L of boric acid, 3-5 g / L of hydrogen peroxide, 3-5 g / L of active carbon, 0.05-0.15 g / L of a surfactant, 0.5-2 g / L of a rear-earth additive, and the balance of deionized water. The nickel plating electrolyte disclosed by the invention is reasonable in formula, not too low in pH value, capable of avoiding separating out much hydrogen on a cathode, high in cathode efficiency, and free of needle holes on the coating; the copper wire nickel-plating electrolyte disclosed by the invention is added with the rear-earth additive, so that dispersion capacity of the nickel-plating liquor is remarkably improved; the rear earth elements have a certain effect on inhibiting hydrogen evolution reaction and can improve current density of the nickel-plating liquor.
Owner:ANHUI NINGGUO TIANCHENG ELECTRICAL APPLIANCES
Preparation method and application of N-heterocyclic carbene modified nickel-iridium diatomic carbon-based catalyst
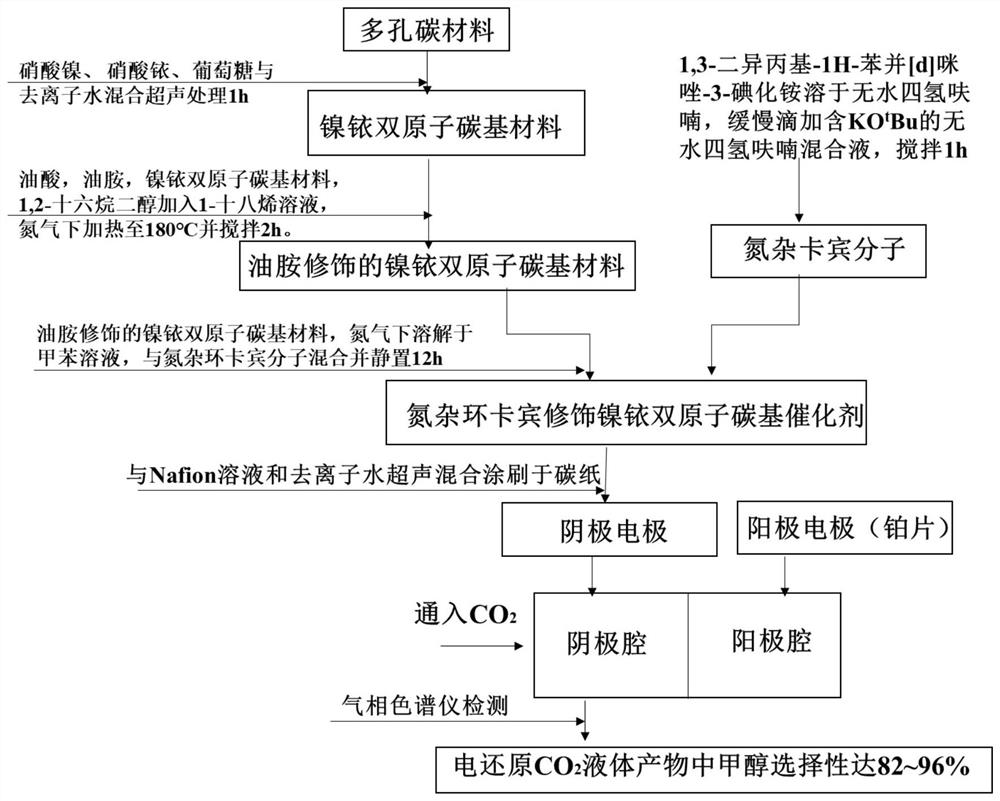
ActiveCN111841641AWell-developed porous structureLarge specific surface areaOrganic-compounds/hydrides/coordination-complexes catalystsElectrolytic organic productionHexadecanePtru catalyst
The invention relates to a conversion and utilization technology of gas CO2, and aims to provide a preparation method and application of an N-heterocyclic carbene modified nickel-iridium diatomic carbon-based catalyst. The preparation method comprises the following steps: pyrolyzing sodium citrate and potassium citrate to obtain a porous carbon material, mixing the porous carbon material with nickel nitrate, iridium nitrate, glucose and deionized water, carrying out ultrasonic treatment, and calcining with melamine in a nitrogen environment; adding the obtained nickel-iridium diatomic carbon-based material, oleic acid, oleylamine and 1, 2-hexadecanediol into a 1-octadecene solution, and carrying out a heating reaction to obtain an oleylamine modified nickel-iridium diatomic carbon-based material; and further reacting with N-heterocyclic carbene molecules to prepare the N-heterocyclic carbene modified nickel-iridium diatomic carbon-based catalyst. The catalyst provided by the inventionhas the remarkable advantages of developed porous structure, large specific surface area, high pyridine nitrogen and pyrrole nitrogen content, strong conductivity and the like; more charges can be provided for CO2 reduction reaction, hydrogen evolution reaction is inhibited due to self hydrophobicity, and high methanol selectivity and carbon atom conversion efficiency are achieved.
Owner:ZHEJIANG UNIV
Preparation method of carbon-based additive used for lead-carbon negative electrode
ActiveCN107742696AIncrease the hydrogen evolution overpotentialInhibition of hydrogen evolution reactionFinal product manufactureLead-acid accumulator electrodesHigh rateState of charge
The invention discloses a preparation method of a carbon-based additive used for a lead-carbon negative electrode, and belongs to the technical field of lead-carbon battery manufacturing. A lead layeron the surface of the carbon-based additive used for the lead-carbon negative electrode prepared through carbon material sensitization, activation and chemical lead plating is uniform and compact indistribution, the hydrogen evolution overpotential of carbon material can be obviously improved, and the hydrogen evolution reaction of the negative electrode of a lead acid battery is inhibited; andthe compatibility of carbon material and lead is promoted, so that a good lead and carbon connecting structure is constructed. The high rate part state-of-charge (HRPSoC) simulation test charge-discharge cycles of the lead acid battery with the negative electrode containing the carbon-based additive used for the lead-carbon negative electrode can reach 10555 circles, and is 11 times of that of a contrast lead acid battery. According to the preparation method, sulfation of the negative electrode of the part state-of-charge (PSoC) can be inhibited obviously, and the cycle life of the lead acid battery is prolonged.
Owner:吉林省凯禹电化学储能技术发展有限公司
Device for photoelectrocatalytic reduction of carbon dioxide
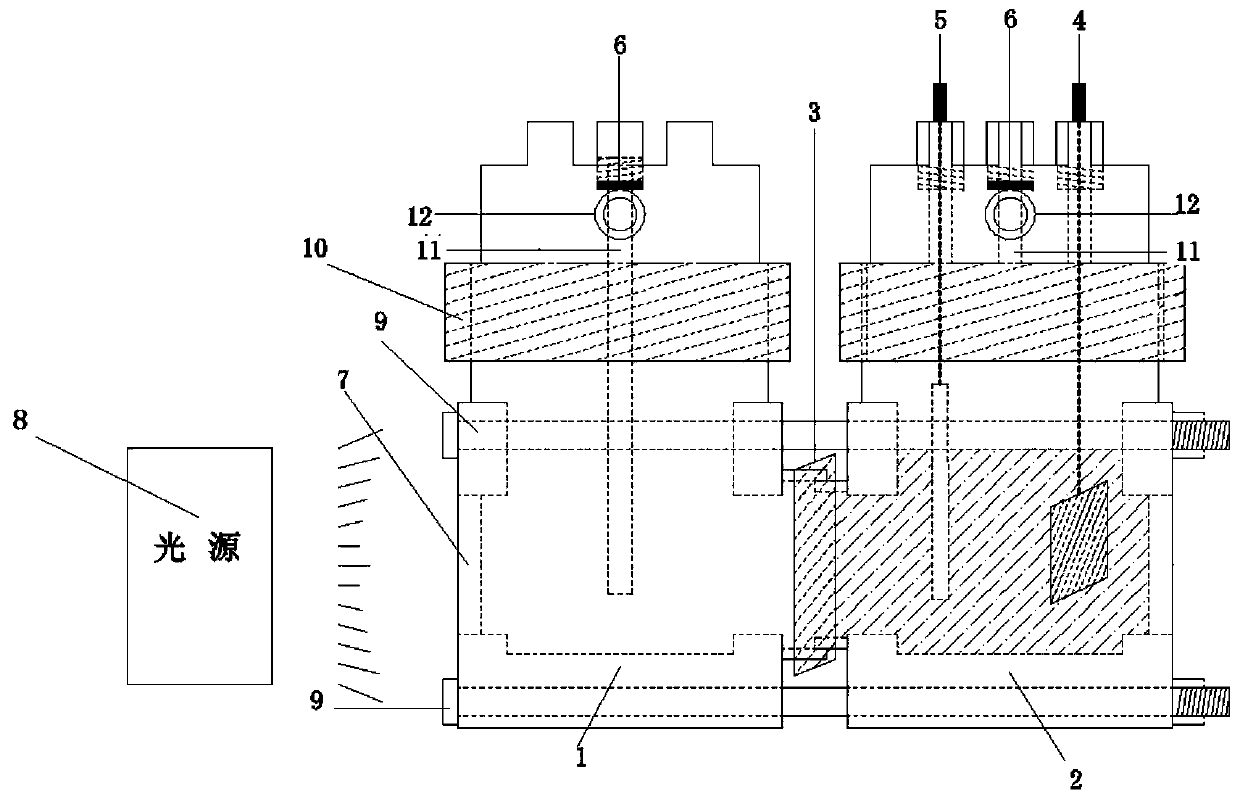
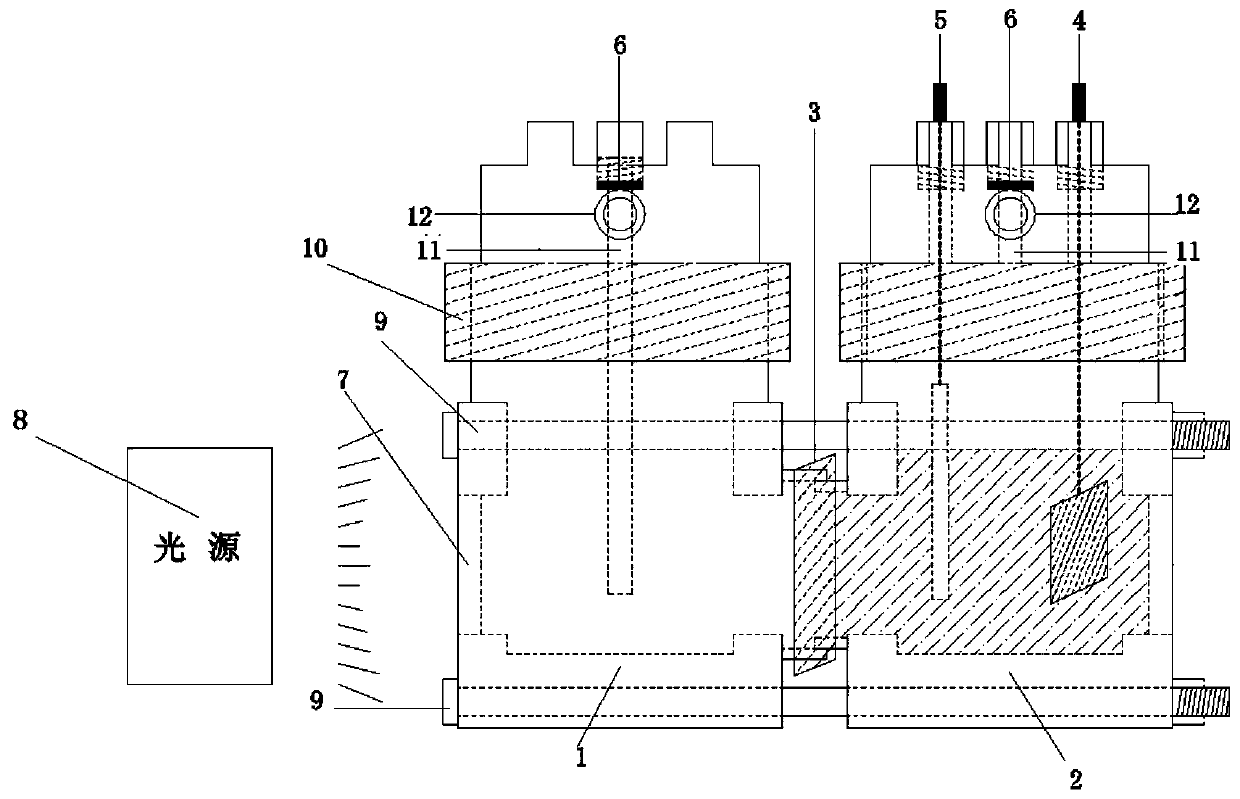
PendingCN110013763AImprove reduction conversion efficiencyImprove sealingGas treatmentDispersed particle separationInjection portPhotocatalytic reaction
The invention discloses a device for photoelectrocatalytic reduction of carbon dioxide. The device comprises a photocatalytic reactor, an electrochemical workstation and a light source lamp arranged outside the photocatalytic reactor; the photocatalytic reactor comprises a photocatalytic reactor, an electrocatalytic reactor, a working electrode, a counter electrode, a reference electrode, a sampleinjection port and a quartz glass window, that photocatalytic reactor and the electrocatalytic reactor are connected through the working electrode, a quartz glass window is arranged on the side of the photocatalytic reactor away from the electrocatalytic reactor, the quartz glass window is correspondingly arranged with the light source lamp, a sample injection port is arranged on the photocatalytic reactor and the electrocatalytic reactor, and the reference electrode and the counter electrode are arranged on the electrocatalytic reactor; the working electrode, the counter electrode and the reference electrode are respectively connected with the electrochemical workstation. The invention can improve the catalytic reduction reaction efficiency of carbon dioxide.
Owner:ZHENGZHOU UNIVERSITY OF LIGHT INDUSTRY
Catalyst for preparing carbon monoxide by electrochemically reducing carbon dioxide and preparation method and application thereof
InactiveCN110090668AAchieving controllable equipmentThe synthesis steps are simpleOrganic-compounds/hydrides/coordination-complexes catalystsElectrodesMass ratioElectrochemistry
The invention relates to a catalyst for preparing carbon monoxide by electrochemically reducing carbon dioxide, and the catalyst comprises a carbon material and a surface organic polymerization layer.The outer surface of the carbon material is coated with the surface organic polymer layer. A non-noble metal is distributed in the surface organic polymer layer. The mass ratio of the surface organicpolymer layer to the carbon nano material is 1: 0.5-1: 4, and the mass fraction of the non-noble metal in the catalyst is 1-10%. Compared with the prior art, a solvent (ethanol / water) used by the invention is more green and environment-friendly, and the preparation process is simpler; the synthesized catalyst has higher catalytic activity, selectivity and stability, provides a new idea for the electrocatalytic efficient conversion of the carbon dioxide, and has a better industrial prospect.
Owner:EAST CHINA UNIV OF SCI & TECH
Preparation method of electric co-depositing zinc magnesium alloy plating layer in aqueous solution
The invention relates to a preparation method of an electric co-depositing zinc magnesium alloy plating layer in an aqueous solution. The preparation method comprises the following steps of: a) preparing an acidic plating solution, preparing a certain amount of zinc sulfate into a solution with a concentration of 100-350g / L, preparing a certain amount of magnesium sulfate into a solution with a concentration of 50-200g / L, and adjusting PH value of the solution to be 1-3 by using sulfuric acid; b) adding polyvinyl glycol as a surface active agent, wherein the concentration of the polyvinyl glycol is 1-4g / L; c) adding complexing agent, namely, adding the complexing agent containing a tartaric acid and sodium monophosphate mixture, wherein the concentration of the tartaric acid is 50-200g / L and the concentration of the sodium monophosphate is 50-150g / L; and d) performing electric deposition by adopting a direct current plating method, thereby lastly obtaining the electric co-depositing zinc magnesium alloy plating layer, wherein the content of magnesium in the plating layer is between 0.8wt% and 2.0wt%. The preparation method provided by the invention has the advantages that the process is stable and is easily operated, and the corrosion resistance of the zinc magnesium alloy plating layer is increased by 3-10 times.
Owner:SHANGHAI JIAO TONG UNIV
Preparation method for NxC-coated metal nanoparticle core-shell-structured material
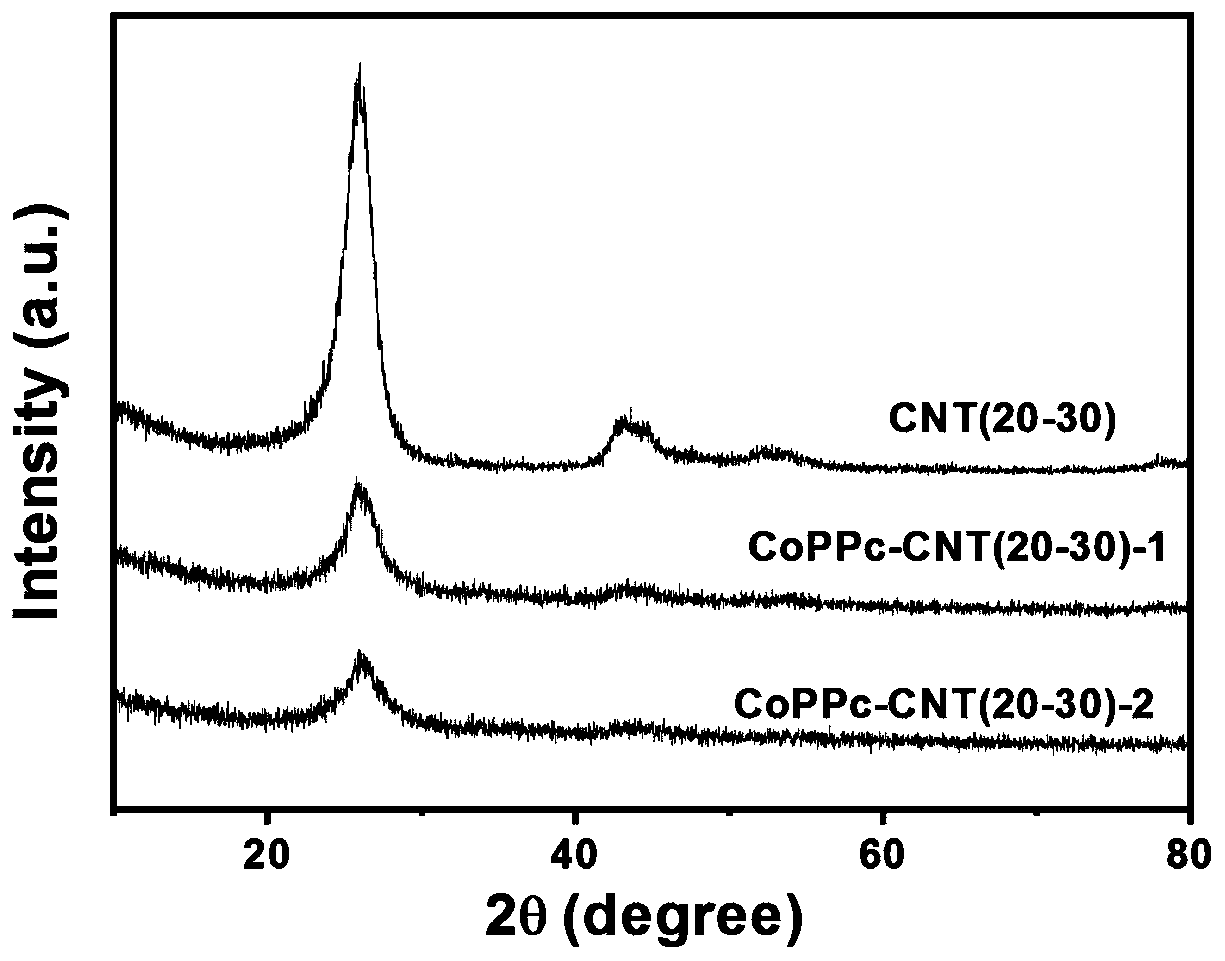
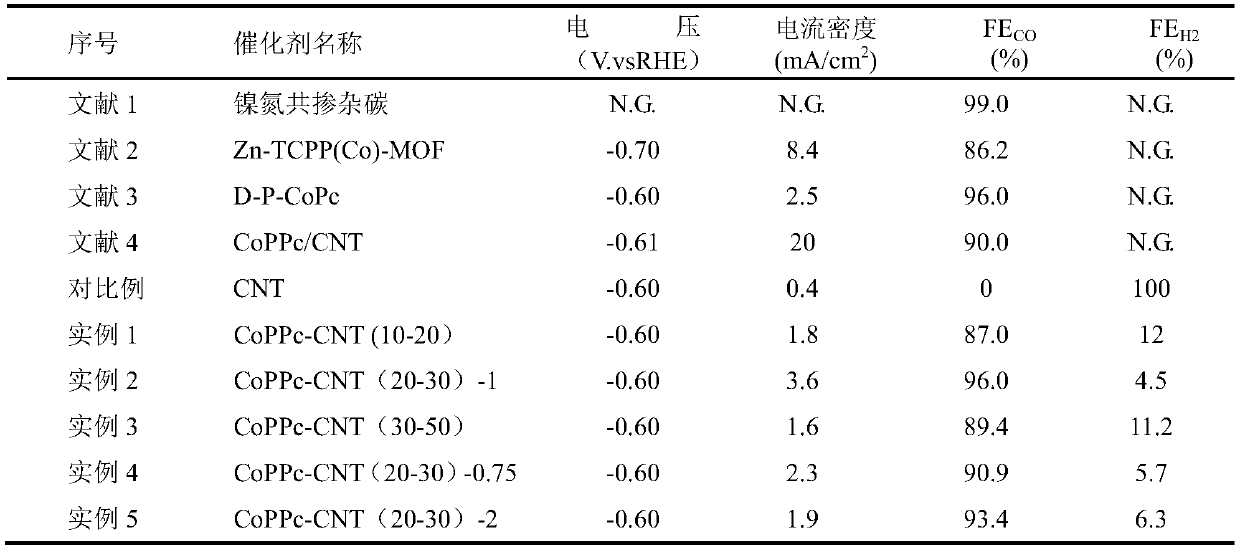
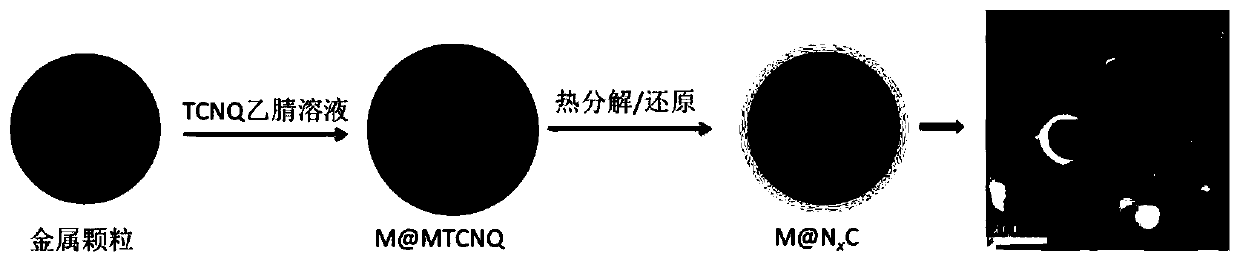
ActiveCN110354881AGood choiceImprove stabilityMaterial nanotechnologyTransportation and packagingAcetonitrileSolvent
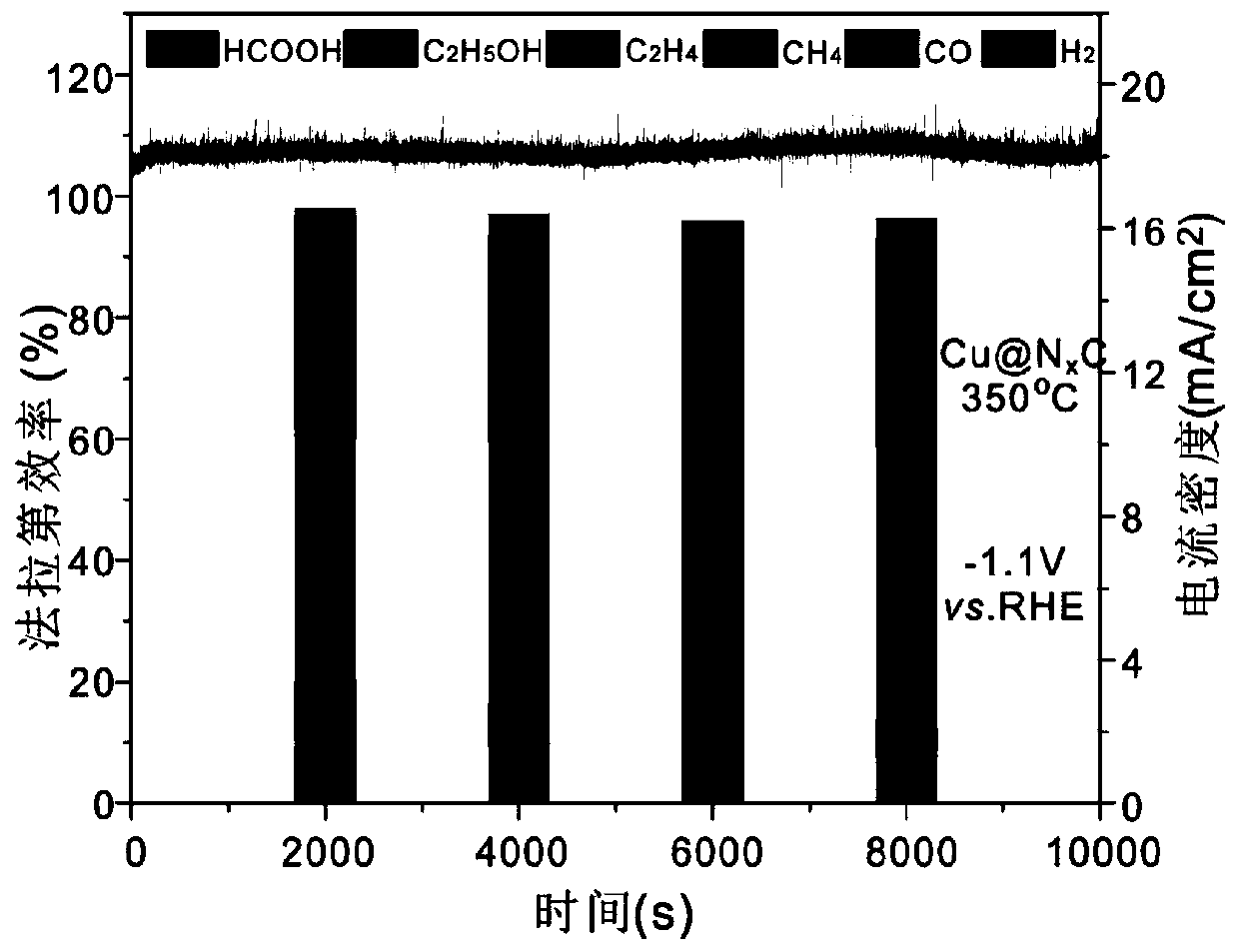
The invention discloses a preparation method for an NxC-coated metal nanoparticle core-shell-structured material. The preparation method comprises the following steps: 1) treating metal nanoparticleswith acid to remove oxides on the surface of the metal nanoparticles; 2) dispersing the obtained nanoparticles in a solvent (such as acetonitrile), slowly adding an organic ligand (such as TCNQ) witha certain mass under stirring, and reacting the ligand with the metal nanoparticles to form an organic salt with a certain thickness on the surfaces of the nanoparticles; and 3) roasting the organic salt-coated metal nanoparticles in a roasting atmosphere to obtain the NxC-coated metal nanoparticle core-shell-structured material. The invention further provides the application of the material. Theinvention provides the preparation method and the application of the NxC-coated metal nanoparticle core-shell-structured material with the metal nanoparticles as a precursor; and the material can be used as a catalyst for a CO2 reduction reaction and has good electrocatalytic activity, good selectivity, high reproducibility and good stability.
Owner:WUHAN UNIV
Boron-nitrogen co-doped carbon material and preparation method and application thereof
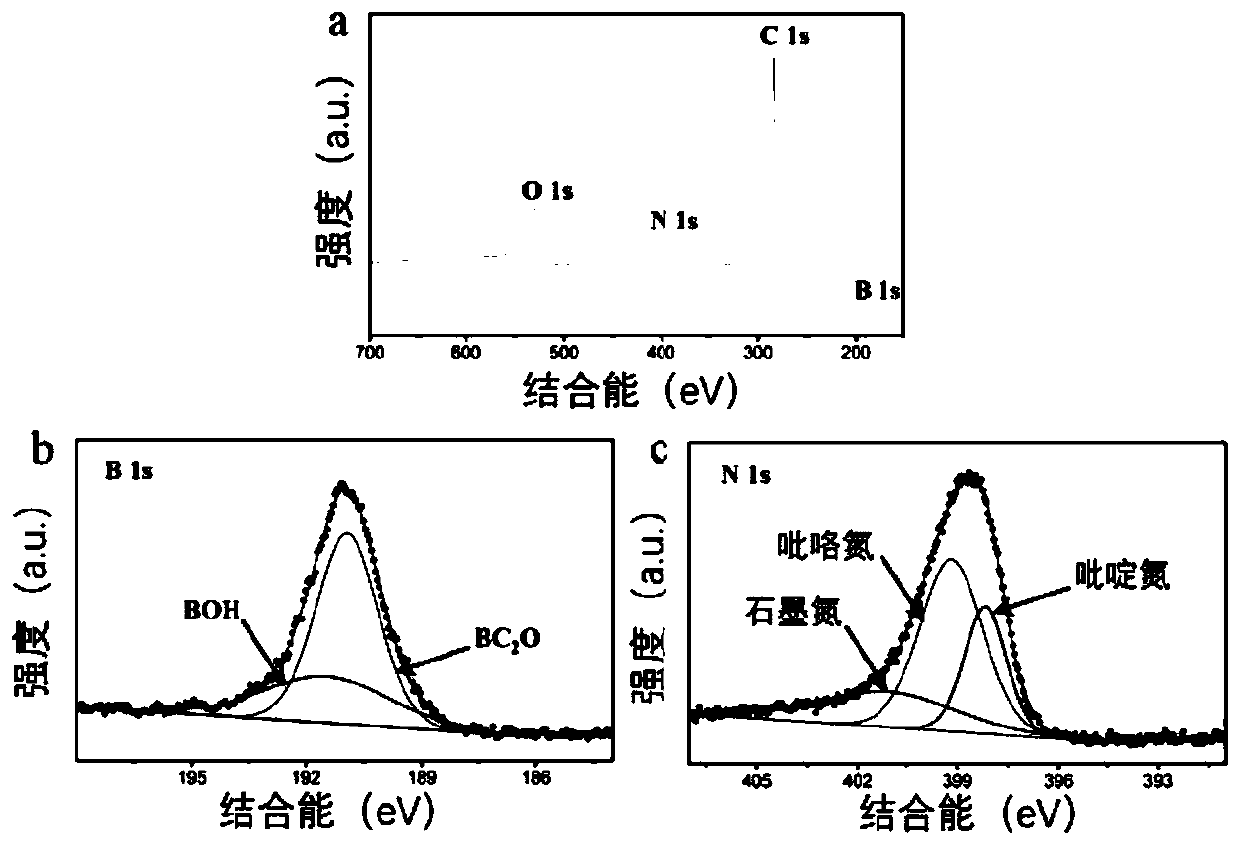
ActiveCN109806898AChange conductivityHigh activityCatalyst activation/preparationElectrodesWater vaporNitrogen
The invention belongs to the technical field of catalyst and ammonia preparation, and discloses a boron-nitrogen co-doped carbon material and a preparation method and application thereof. The method comprises the following steps: respectively placing boron nitride and a carbon material in different high temperature reaction regions of a reaction device, wherein boron nitride is located above a gasflow, and the carbon material is located below the gas flow; under the flow of carrier gas, transporting water vapor to a region where the boron nitride is located, and enabling boron nitride to react with the water vapor at a high temperature to obtain a precursor small molecule; transporting the precursor small molecule along with the gas flow to a region where the carbon material is located, and enabling the carbon material and the precursor small molecule to make a co-doping reaction to obtain the boron-nitrogen co-doped carbon material; the temperature of the high temperature reaction is800 to 1200 DEG C; the temperature of the co-doping reaction is 500 to 900 DEG C. The method is simple and low-cost, adopts the raw materials which are cheap and easy to obtain, and is environmentally friendly. The prepared boron-nitrogen co-doped carbon material has good catalytic efficiency when catalyzing nitrogen to prepare ammonia. The boron-nitrogen co-doped carbon material is used for catalyzing nitrogen to produce ammonia.
Owner:SOUTH CHINA UNIV OF TECH
Anode alloy material for magnesium air battery, preparation method thereof and battery
ActiveCN111793760AIncrease the hydrogen evolution overpotentialIncrease discharge voltageFuel and primary cellsCell electrodesSolution treatmentMetallurgy
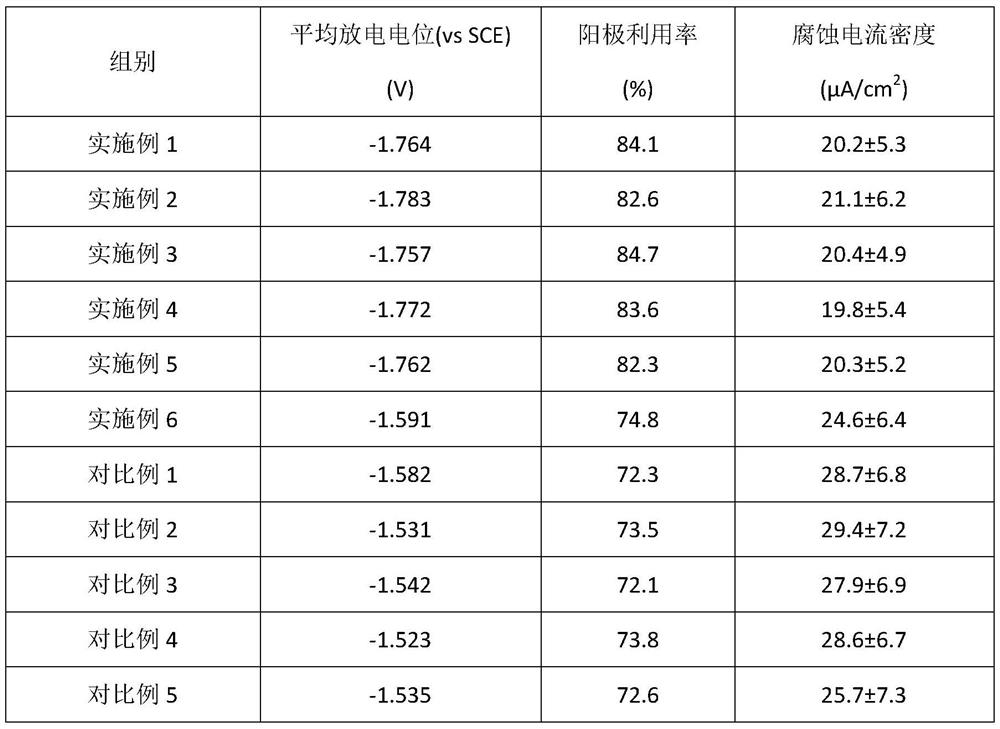
The invention discloses an anode alloy material for a magnesium-air battery, a preparation method thereof, and a battery, and relates to the technical field of materials. The anode alloy material formagnesium air battery is prepared from the components in percentage by mass: 1.5% to 11.5% of Al, 0.5% to 6.5% of Ca, 0.2% to 6.0% of Bi, 0.1% to 4.0% of Ce, the limiting elements Fe less than or equal to 0.01%, Cu less than or equal to 0.01%, Ni less than or equal to 0.01%, and the balance of Mg. The preparation method of the anode alloy material for the magnesium air battery comprises the stepsof solid solution treatment, rolling, and annealing on casting blanks. The content of each element contained in the casting blanks is matched with the element content of the anode alloy material to beprepared. The anode alloy material can increase the hydrogen evolution overpotential of the magnesium anode, effectively inhibit the hydrogen evolution reaction. The material can make discharge potential relatively negative and the anode utilization rate high. The anode of the battery is made of the alloy materials prepared by the preparation method, and the performance of the alloy materials isgood.
Owner:GUANGDONG INST OF NEW MATERIALS
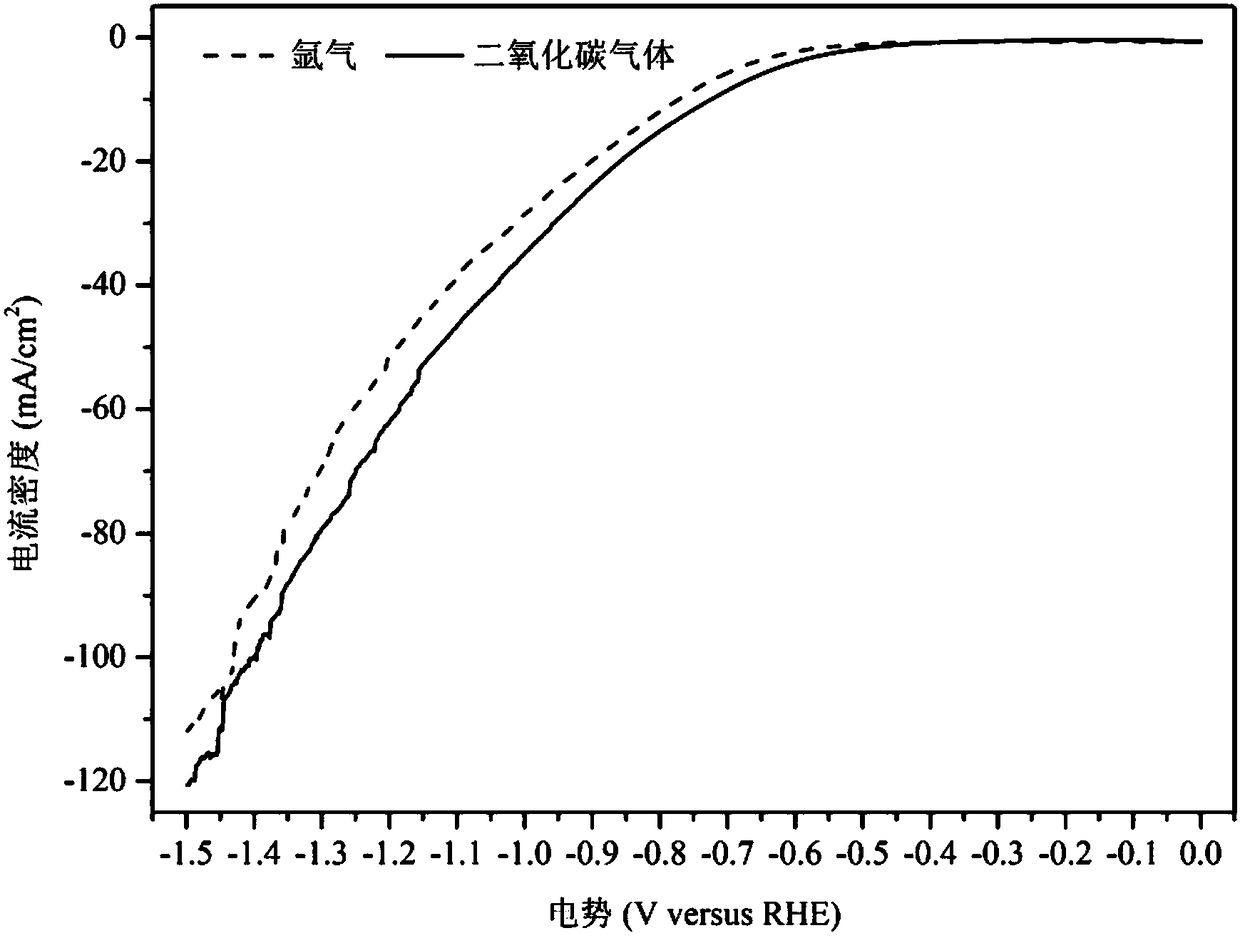
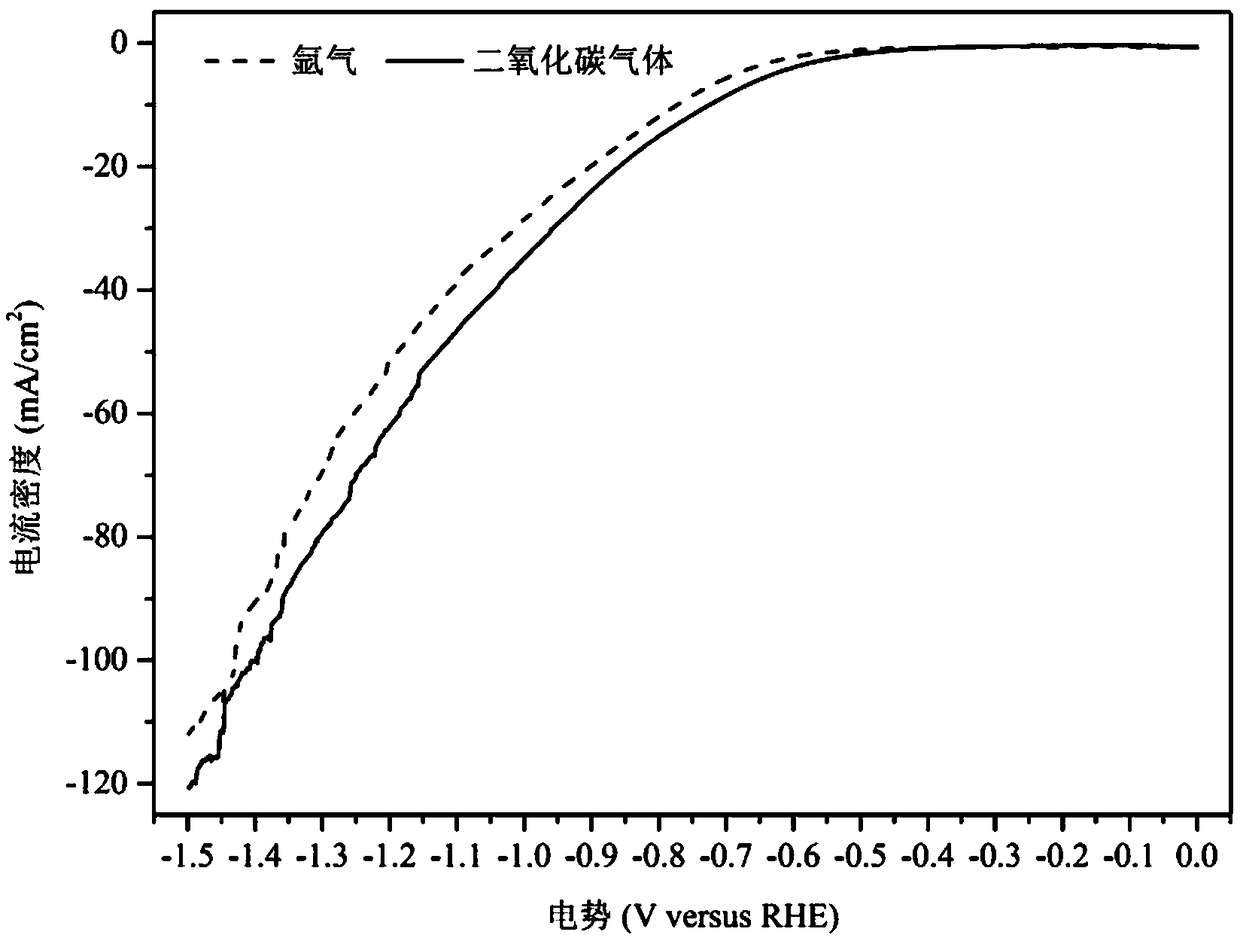
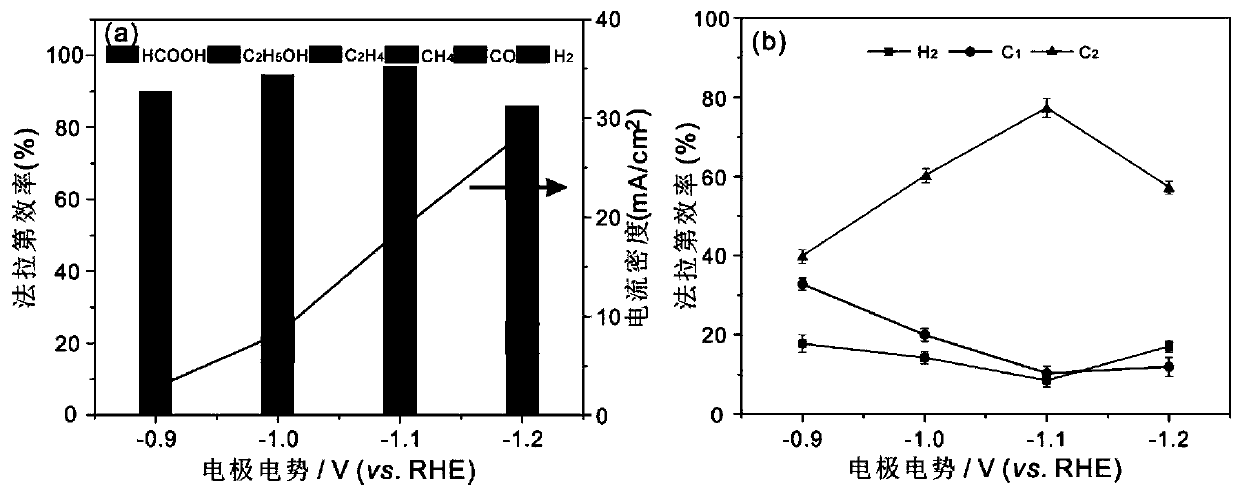
Method for preparing carbon monoxide through carbon dioxide electrochemical reduction
ActiveCN112251766AIncrease the catalytic activity of electrochemical reductionImprove Faraday efficiencyCellsElectrodesPtru catalystFaraday efficiency
The invention relates to a method for preparing carbon monoxide by electrochemical reduction of carbon dioxide. The method comprises the following steps of: separating a cathode chamber and an anode chamber by a proton exchange membrane by adopting an H-shaped double-electrochemical-cell reactor; introducing carbon dioxide gas into the cathode chamber before reaction; and using a three-electrode system, wherein a gas diffusion electrode is used as a working electrode, a platinum electrode is used as an auxiliary electrode, a silver / silver chloride electrode is used as a reference electrode, the gas diffusion electrode comprises a gas diffusion electrode body and a carbon dioxide electrochemical reduction catalyst loaded on the gas diffusion electrode body, and the carbon dioxide electrochemical reduction catalyst is gold-based bimetal supported by multi-walled carbon nanotubes. The method can improve the Faraday efficiency of the product carbon monoxide.
Owner:SINOPEC NANJING RES INST OF CHEM IND CO LTD +2
Boron-doped diamond supported metal single atom and preparation method and application thereof
ActiveCN110560034AImprove Faraday efficiencyImprove atom utilization efficiencyCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsMetallurgyElectrochemical window
The invention provides a boron-doped diamond supported metal single atom. The boron-doped diamond supported metal single atom comprises a substrate, a boron-doped diamond thin film arranged on one ortwo sides of the substrate, and a catalyst uniformly supported on the surface of the boron-doped diamond thin film, wherein the catalyst comprises a metal single atom. A prepared electrode of the boron-doped diamond supported metal single atom has a wide electrochemical window, high stability, and high catalytic activity and product selectivity, so that selectivity and efficiency of a catalytic process are greatly improved.
Owner:SHENZHEN INST OF ADVANCED TECH
Aqueous zinc ion battery electrolyte additive
ActiveCN112635860AImprove cycle lifeAchieve full coverageEnergy storageSecondary cells servicing/maintenanceElectrolytic agentZinc ion
The invention discloses a zinc ion battery electrolyte additive. The additive is amino acids, lipopeptides or polypeptides; an electrolyte is a zinc salt electrolyte, i.e., an electrolytezinc formed by dissolving salt is in deionized water; the concentration of the zinc salt in the electrolyte is 1-3mol / L; and the concentration of the additive added in the electrolyte is 0.01-0.5 mol / L. The additive disclosed by the invention contains alkaline amino acid residues, and can be in a positively charged state in a weakly acidic electrolyte; and the positively charged amino acids, lipopeptides or polypeptides can be adsorbed to favorable nucleation sites of zinc to promote uniform deposition of zinc ions in a zinc deposition process, so that full coverage of the favorable nucleation sites is realized, and dendritic crystal-free growth is realized. The additive has the advantages of inorganic ions and organic polymer additives at the same time, so that dendritic crystal-free growth and corrosion inhibition of a zinc negative electrode are realized, and the cycle life of a battery is prolonged.
Owner:ZHEJIANG UNIV
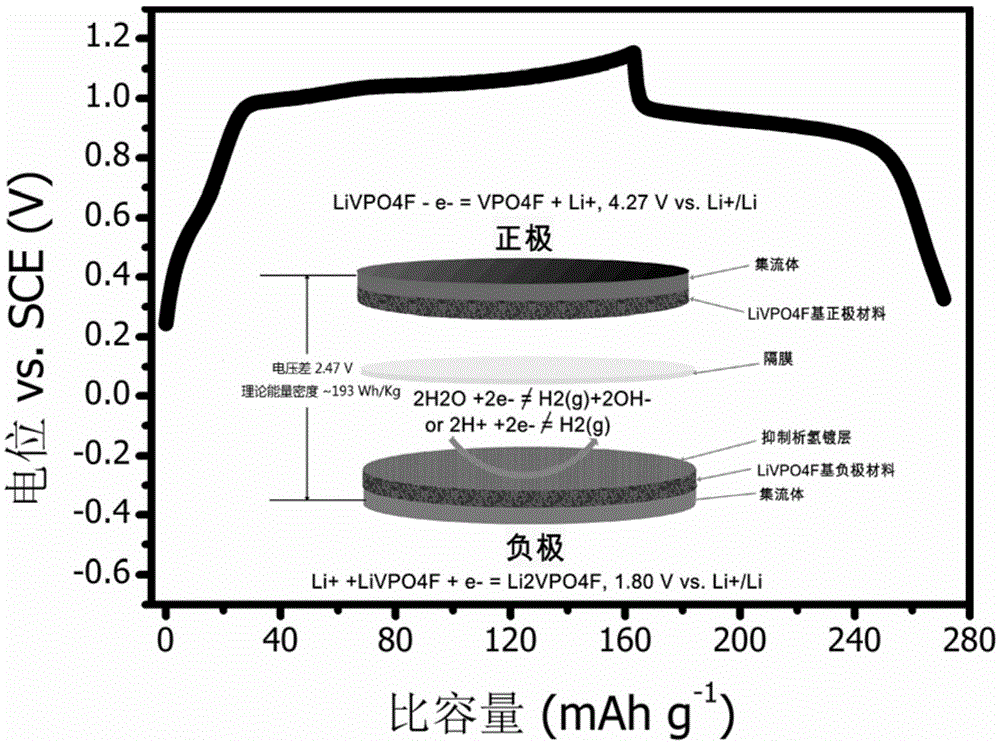
A symmetrical aqueous solution lithium-ion battery
ActiveCN103928681BIncrease energy densityImprove power densityElectrode carriers/collectorsSecondary cellsElectrical batteryLithium-ion battery
The invention discloses a symmetric aqueous solution lithium ion battery and a modification method. Fluorine lithium vanadium phosphate is simultaneously used as a positive electrode and a negative electrode, and an aqueous solution containing lithium ions serves as electrolyte. An electrode surface serving as the negative electrode is covered with a protection film which is 5-1,000nm thick and has high hydrogen evolution overpotential so as to suppress hydrogen evolution of the negative electrode. According to the symmetric aqueous solution lithium ion battery, the fluorine lithium vanadium phosphate is simultaneously applied to the positive electrode and the negative electrode of water system lithium ions, so that the manufacturing cost of an electrode piece material is reduced. The symmetric aqueous solution lithium ion battery modified by the modification method disclosed by the invention effectively suppresses hydrogen evolution reaction and has the characteristics of long service life, high power, high safety, no environment pollution and the like.
Owner:湖南豪曼新能源科技有限公司
Anode alloy material, preparation method thereof, anode for aluminum-air battery and aluminum-air battery
ActiveCN111057914AInhibition of hydrogen evolution reactionIncrease profitFuel and secondary cellsCell electrodesMetallurgyBattery cell
The invention discloses an anode alloy material, a preparation method thereof, an anode for an aluminum-air battery and the aluminum-air battery, and relates to the field of anode materials for air batteries. The anode alloy material comprises aluminum and alloy elements added into the aluminum, wherein the alloy elements comprise, by mass, 0.01%-5.5% of Mg, 0.01%-3.0% of Sn, 0.01%-2.5% of Ce, 0.0001%-0.0010% of B and 0.01%-2.0% of Ti. The preparation method comprises the steps of preparing the anode alloy material from the raw materials according to the component matching ratio. The components of the anode alloy material are limited, the dosage of each component of the anode alloy material is adjusted, the optimal component dosage ratio is obtained, the hydrogen evolution reaction of thealuminum alloy anode material can be effectively inhibited, and the anode alloy material with negative average discharge potential and high anode utilization rate is developed.
Owner:GUANGDONG INST OF NEW MATERIALS
Water-ether mixed electrolytic solution for lithium ion battery, and preparation method thereof
ActiveCN111477977ALow viscosityImprove ionic conductivitySecondary cellsAqueous electrolytesElectrolytic agentElectrical battery
The invention relates to a water-ether mixed electrolytic solution for a lithium ion battery, and a preparation method thereof, and belongs to the technical field of lithium secondary batteries. According to the invention, the mixed electrolytic solution is obtained by mixing a lithium salt, water and an ether solvent, and has relatively low viscosity, relatively high ionic conductivity and a relatively wide electrochemical stability window; the mixed electrolytic solution and an electrode material can generate a compact SEI film, so that a positive electrode and a negative electrode are protected against corrosion, normal charging and discharging of a common commercial electrode material can be guaranteed, and the electrochemical performance of the common commercial electrode material isimproved; and the preparation process of the mixed electrolytic solution is simple, the raw materials are easy to obtain, safe and pollution-free, and the mixed electrolytic solution is suitable for large-scale batch production.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Method for increasing manganese leaching rate in low-grade manganese ore
ActiveCN110684900AImprove leaching rateImprove adsorption capacityPhotography auxillary processesElectrolytic agentActive agent
The invention provides a method for increasing the manganese leaching rate in low-grade manganese ore. In the low-grade manganese ore leaching process, a proper amount of surfactant is added, the surface activity of mineral particles is improved, the adsorption effect of the ore particles on hydrogen ions is improved, oily organic matter is effectively removed, and meanwhile, the manganese leaching rate is improved. In the filter pressing process of a manganese ore leaching solution, the surfactant is continuously filtered away, so that the surface tension of the manganese ore leaching solution is continuously decreased, and the current efficiency of the manganese ore leaching solution tends to be decreased after rising. In the low-grade manganese ore electrolysis process, a proper amountof surfactant is added, so that the surface tension value of the manganese ore leaching solution is optimal, and the current efficiency of electrolytic manganese is remarkably improved. The redox potential of electrolyte is reasonably controlled, so that the current efficiency of the solution is highest. Selenium dioxide with a certain concentration is added into the electrolyte during electrolysis so that occurrence of hydrogen evolution reaction can be inhibited, the current efficiency of the electrolytic manganese process can be remarkably improved, the energy consumption of the electrolytic manganese is low, and the manganese leaching rate is high.
Owner:广西大新汇元新能源科技有限责任公司
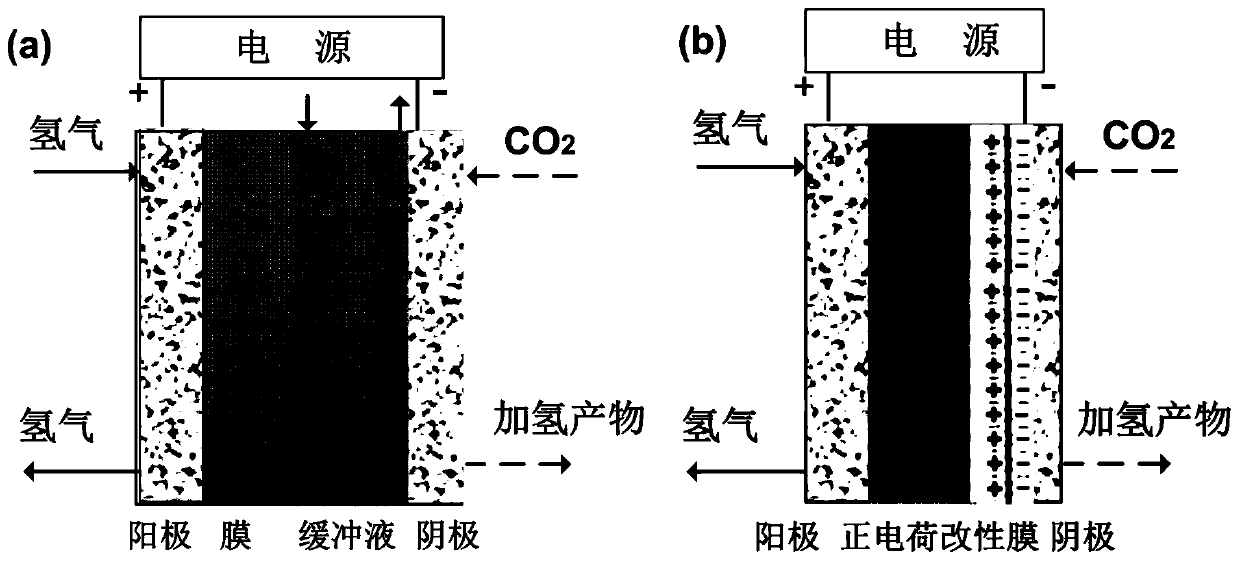
Method for controlling cathode potential in electrochemical hydrogen pump CO2 hydrogenation reactor by film method
ActiveCN110311161AHigh cathodic potentialAvoid liquid environmentFuel cellsHydrogenation reactionProton
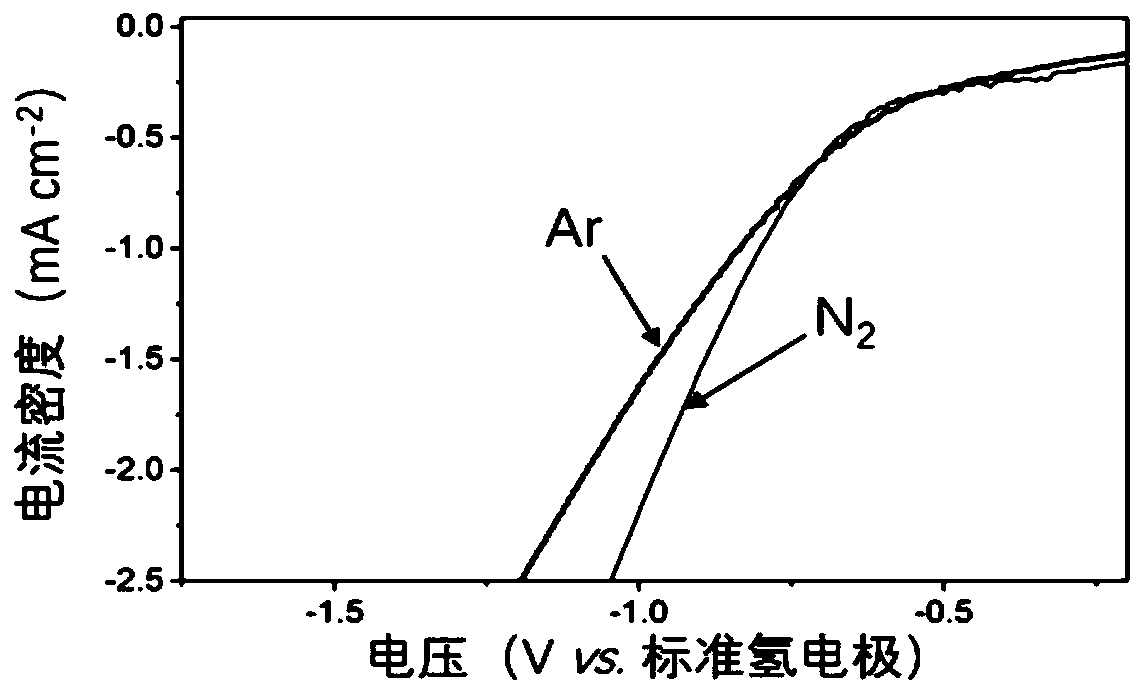
The invention belongs to the technical field of electrochemical engineering and relates to a method for controlling the cathode potential in an electrochemical hydrogen pump CO2 hydrogenation reactorby a film method. The method is characterized in that a positive charge-modified proton exchange film is utilized in place of a liquid buffer layer, when electric energy is applied to the electrochemical hydrogen pump CO2 hydrogenation reactor, hydrogen protons generated by anode hydrogen dissociation pass through the positive charge-modified proton exchange film, and in-situ adsorbed hydrogen generated at a cathode undergoes hydrogenation reaction with CO2; positive charges are introduced into the proton exchange film through ion displacement or layer-by-layer self-assembling, the positive charges in the film migrate and accumulate toward the cathode under the action of an electric field to form a double electrode layer with the cathode, the cathode potential of CO2 hydrogenation is regulated, and CO2 hydrogenation reaction is promoted. The method is advantaged in that the modified film is utilized in place of the liquid buffer layer, the liquid phase environment can be avoided, interface problems are eliminated, the higher cathode potential can be achieved, moreover, the Donnan repulsion effect of the positive charges to the hydrogen protons is utilized, the hydrogen evolution reaction is stably inhibited for a long time, and relatively high CO2 hydrogenation efficiency is achieved.
Owner:DALIAN UNIV OF TECH
Alloy anode material for aluminum-air battery, preparation method thereof and aluminum-air battery
ActiveCN110931812ALow discharge potentialHigh anode utilizationFuel and primary cellsCell electrodesMetallurgyElectrical battery
The invention relates to the technical field of electrochemistry, and discloses an alloy anode material for an aluminum-air battery, a preparation method of the alloy anode material and the aluminum-air battery. The alloy anode material for the aluminum-air battery comprises the following chemical components in percentage by mass: 0.01 to 2.0 percent of Pb, 0.01 to 2.5 percent of Bi, 0.01 to 1.5 percent of Ga, 0.01 to 2.0 percent of Li, 0.0001 to 0.0010 percent of B and the balance of Al. The preparation method of the alloy anode material for the aluminum-air battery comprises the step of preparing the alloy anode material for the aluminum-air battery by adopting the raw materials containing the chemical components. When the alloy anode material for the aluminum-air battery is used, the hydrogen evolution reaction on the surface is improved, the discharge potential is low, and the anode utilization rate is high. The aluminum-air battery comprises the alloy anode material for the aluminum-air battery, and is good in electrochemical performance.
Owner:GUANGDONG INST OF NEW MATERIALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com