Carbon dioxide electrochemical-reduction catalyst, and preparation and application thereof
A carbon dioxide and catalyst technology, which is applied to the electrochemical reduction of carbon dioxide catalyst and the fields of preparation and application thereof, can solve the problems of unreported electrochemical reduction, and the effect of electrochemical reduction has not received extensive attention, and achieves reduction of catalyst deactivation effect, Good application prospects and the effect of improving current efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
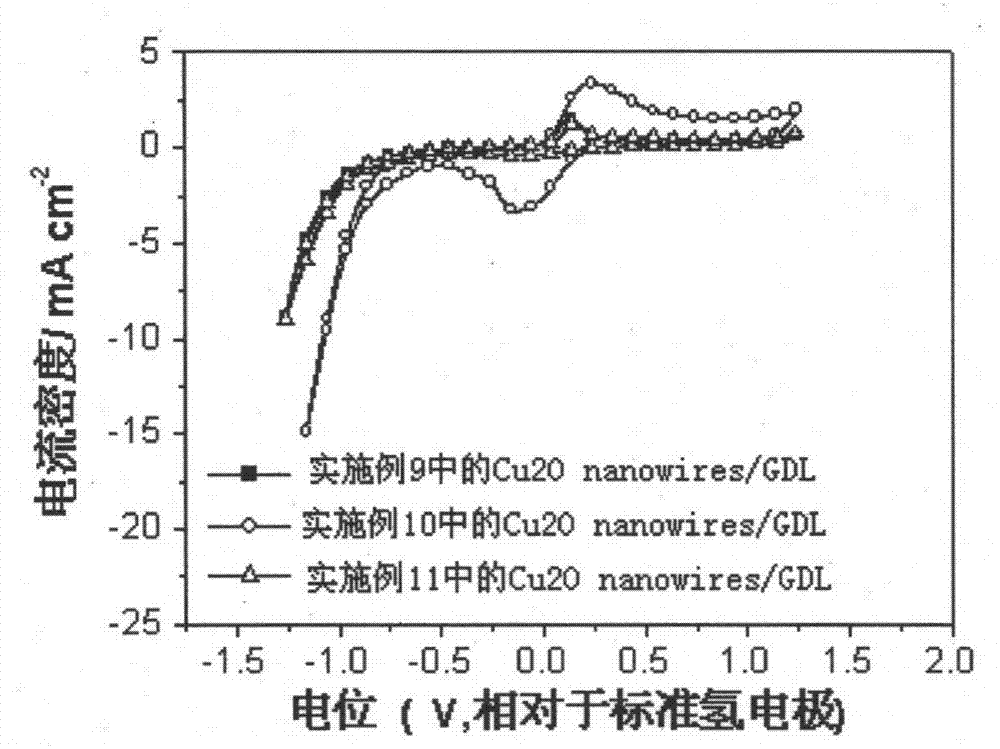
[0025] Cu 2 Preparation of O nanowires catalyst precursor: Dissolve 0.02mol copper acetate and 0.002mol 2,5-dimethoxyaniline in 500mL and 100mL deionized water, respectively, to prepare 0.04M copper acetate solution and 0.02M methoxyaniline solution . Take 40mL0.04M copper acetate solution and 10mL0.02M methoxyaniline solution and mix thoroughly to obtain Cu 2 O nanowires catalyst precursor, and transferred to a 100 mL reactor.
Embodiment 2
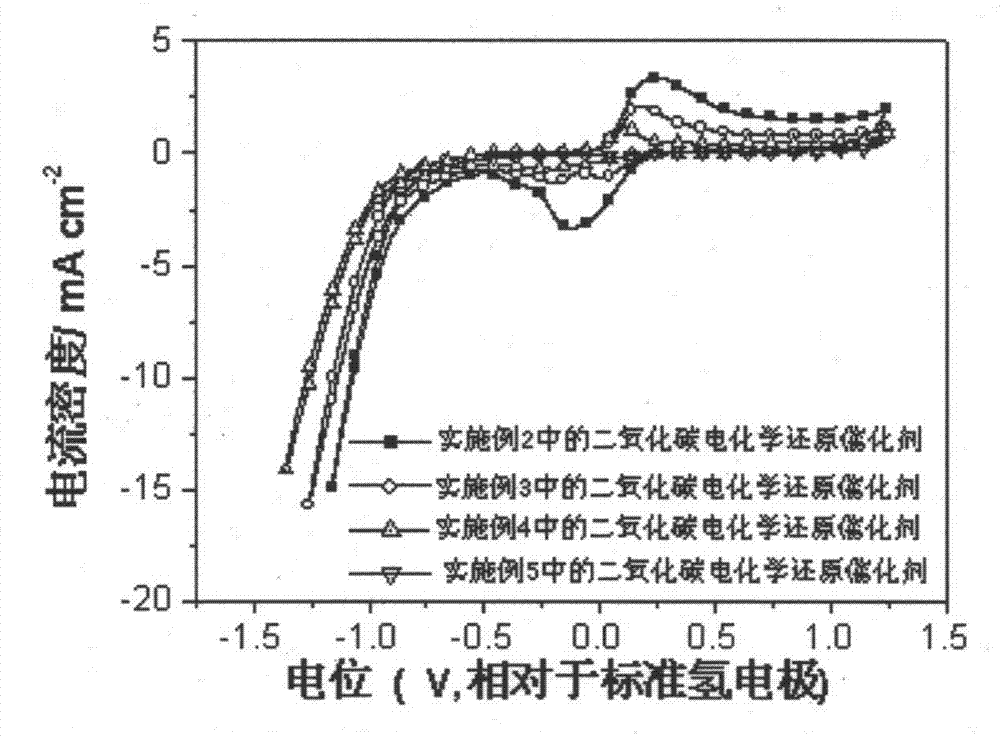
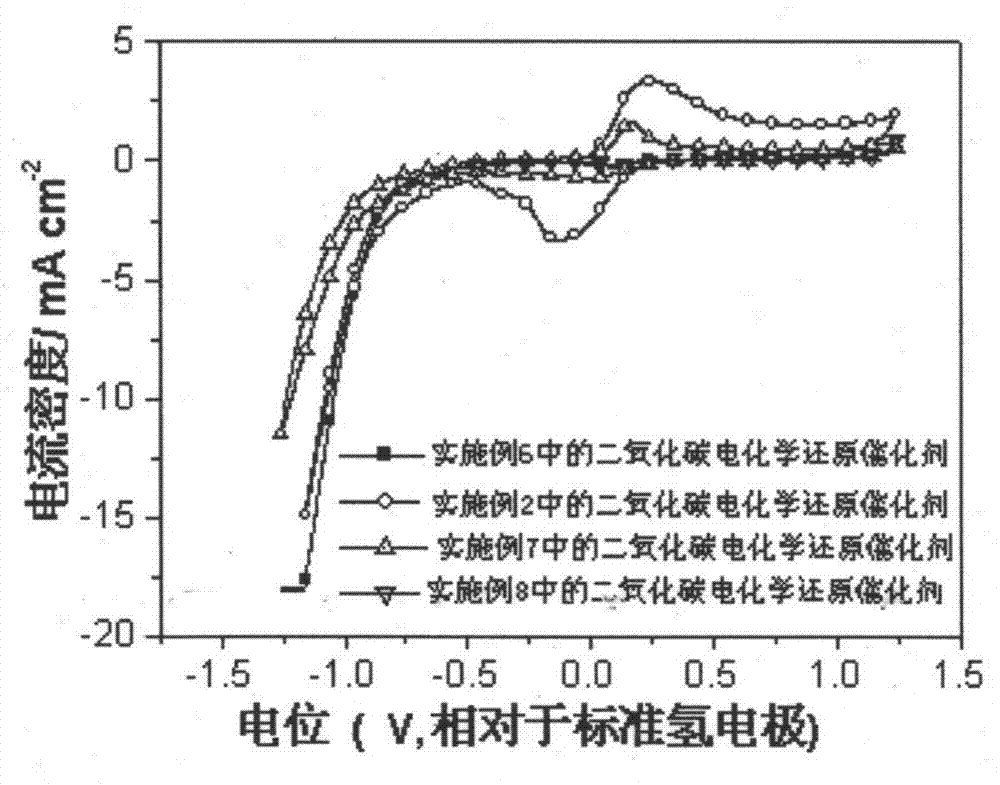
[0027] A carbon dioxide electrochemical reduction catalyst, including cuprous oxide nanowires, the cuprous oxide nanowires are synthesized by hydrothermal reaction, the preparation method is: take 0.02mol copper acetate and 0.002mol2,5-dimethoxy Aniline was dissolved in 500mL and 100mL of deionized water respectively, and respectively made into 0.04M copper acetate solution and 0.02M methoxyaniline solution. Take 40mL of 0.04M copper acetate solution and 10mL of 0.02M methoxyaniline solution and mix thoroughly to obtain a catalyst precursor, and move the catalyst precursor into a 100mL reactor. Hydrothermal reaction kettle, put the reaction kettle into an oven at 180°C for hydrothermal reaction for 2 hours, centrifuge, wash with ethanol, and dry to obtain cuprous oxide nanowires, which are carbon dioxide electrochemical reduction catalysts, called Cu 2 O nanowires-2h catalyst.
Embodiment 3
[0029]A carbon dioxide electrochemical reduction catalyst, including cuprous oxide nanowires, the cuprous oxide nanowires are synthesized by hydrothermal reaction, the preparation method is: take 0.02mol copper acetate and 0.002mol2,5-dimethoxy Aniline was dissolved in 500mL and 100mL of deionized water respectively, and respectively made into 0.04M copper acetate solution and 0.02M methoxyaniline solution. Take 40mL of 0.04M copper acetate solution and 10mL of 0.02M methoxyaniline solution and mix thoroughly to obtain a catalyst precursor, and move the catalyst precursor into a 100mL reactor. Hydrothermal reaction kettle, put the reaction kettle in an oven for hydrothermal reaction at 180°C for 5 hours, centrifuge, wash with ethanol, and dry to obtain cuprous oxide nanowires, which are carbon dioxide electrochemical reduction catalysts, called Cu 2 O nanowires-5h catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com