Method for preparing NiS/Ni(OH)2 electrocatalyst used for decomposing water to generate hydrogen
An electrocatalyst and water hydrogen production technology, which is applied in the direction of electrodes, electrolysis process, electrolysis components, etc., can solve the problems of complicated process and harsh operating conditions, and achieve the effects of simple operation, high reaction efficiency, and simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
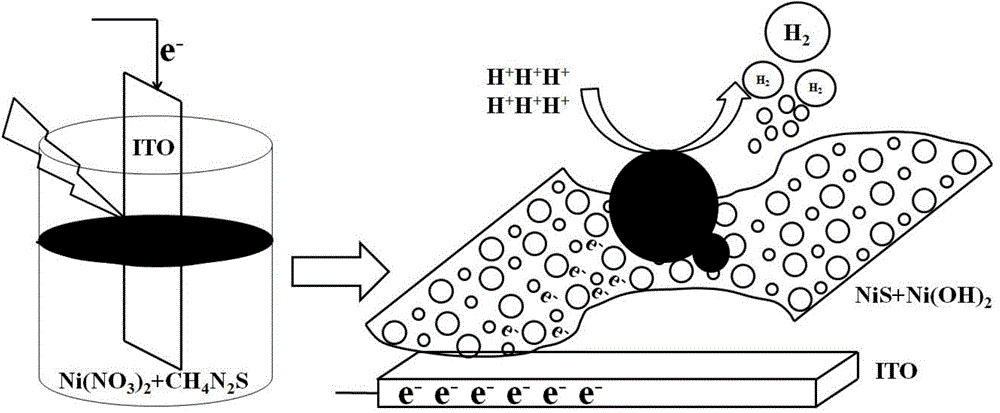
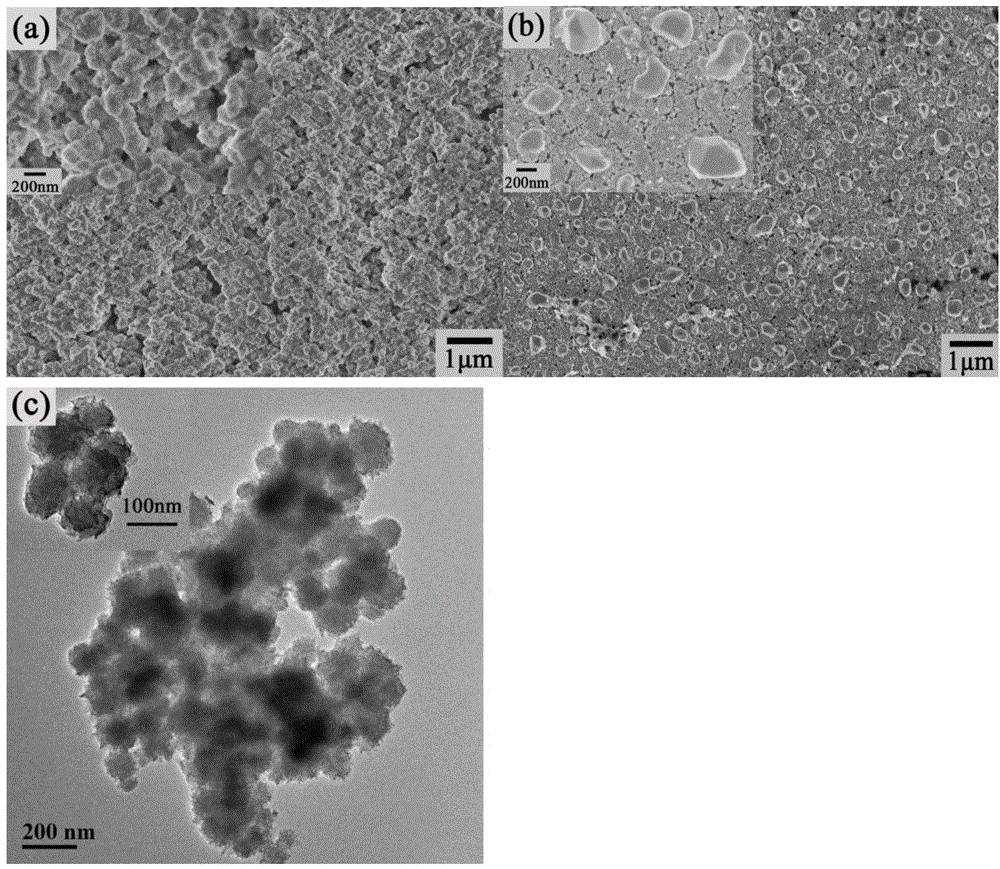
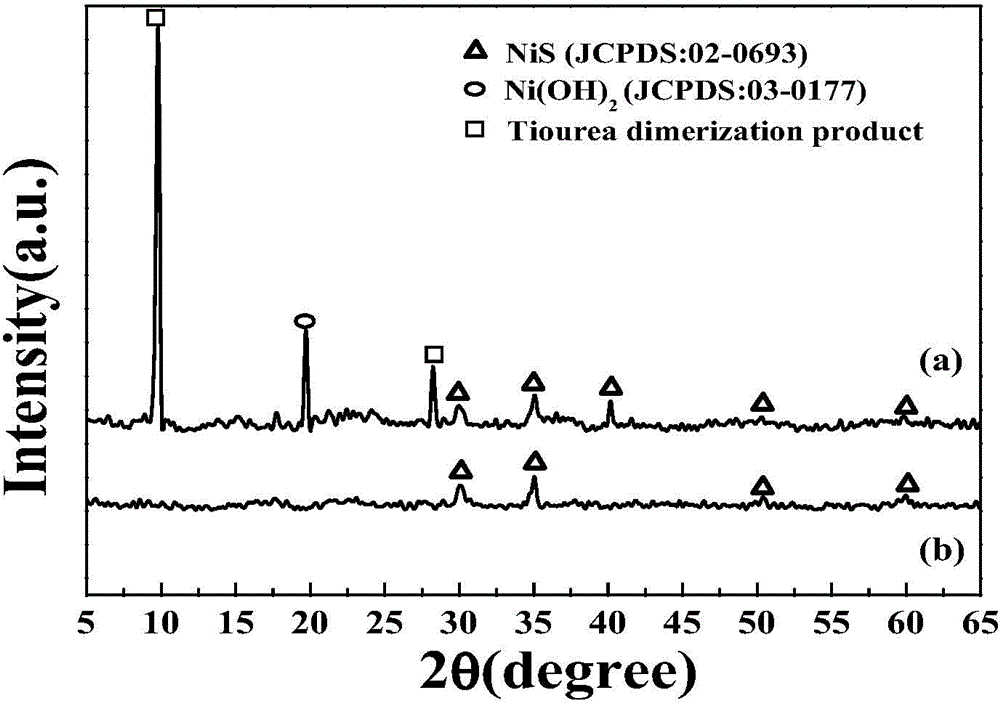
[0028] NiS / Ni(OH) 2 The preparation method of electrocatalyst, its concrete steps are: (1) the surface pretreatment of ITO conductive glass: ITO conductive glass is first successively soaked in dehydrated alcohol, acetone for two hours, and then in the isotropic solution of 2mol / L potassium hydroxide Heating to reflux in propanol solution for 30min. (2) Light-assisted electrokinetic deposition of NiS / Ni(OH) 2 As an electrocatalyst: Deposition of NiS / Ni(OH) on ITO electrodes by light-assisted electrokinetic deposition 2 , first configure the deposition solution, that is, 5Mm NiCl 2 ·6H 2 O and 0.3M thiourea solution. With ITO as the working electrode, Ag / AgCl (0.3M) as the reference electrode, and platinum mesh as the counter electrode, the working electrode was placed in the deposition solution, and the reference and counter electrodes were placed in Na 2 SO 4 middle. Photoassisted electrokinetic deposition was performed with cyclic voltammetry at a scan rate of 5 mV s ...
Embodiment 2
[0031] Deposition of NiS / Ni(OH) with different layers on ITO by light-assisted electrokinetic deposition 2 , to investigate its electrochemical performance under the same conditions. Using light-assisted electrokinetic deposition, 1, 3, 6, and 9 layers of Ni-S were deposited on cleaned ITO glass, respectively, and blank ITO and Pt were used as controls. The light source used is a xenon lamp (400nm), the electrochemical deposition technique is cyclic voltammetry, and the scan rate is 5mV s -1 , The scanning voltage range is -1.2V~0.2V, and the deposition solution used is a mixture of inorganic nickel salt and thiourea. The prepared electrode is used as a working electrode, Ag / AgCl (0.3M) is a reference electrode, a platinum mesh is a counter electrode, placed in a buffer solution of pH=6, and the buffer solution contains hexamethylenetetramine / hydrochloric acid Buffered KCl solution, before use with CH 4 / N 2 The mixed gas was degassed and deoxygenated, and then used to mea...
Embodiment 3
[0034] Made NiS / Ni(OH) 2 Electrode hydrogen production efficiency evaluation.
[0035] Use a mixed aqueous solution (pH=6) of 0.30mol / L hexamethylenetetramine, 0.10mol / L hydrochloric acid and 0.20mol / L potassium chloride as the electrolyte solution, and use CH 4 / N 2 The mixed gas is used for degassing and oxygen removal, the Ag / AgCl electrode is used as the reference electrode, and the platinum mesh is used as the counter electrode. The prepared NiS / Ni(OH) 2 The electrode was used as the working electrode, and the electrohydrogen experiment was done on the CHI600D electrochemical instrument. The amount of photocatalytic hydrogen production was detected by gas chromatography with a thermal conductivity detector. Electrode NiS / Ni(OH) of the present invention 2 The electrocatalytic hydrogen production efficiency under different conditions is shown in the figure.
[0036] From Figure 6 It can be seen that the hydrogen content measured by gas chromatography is roughly consi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com