Electrode for electrochemical reduction of carbon dioxide, as well as preparation method and application of electrode
A carbon dioxide and electrochemical technology, applied in the direction of electrodes, electrolytic processes, electrolytic components, etc., can solve the problems of cumbersome electrode production process, complicated preparation method, and decreased activity ratio, so as to improve the effective active area, simple preparation method, and simple method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
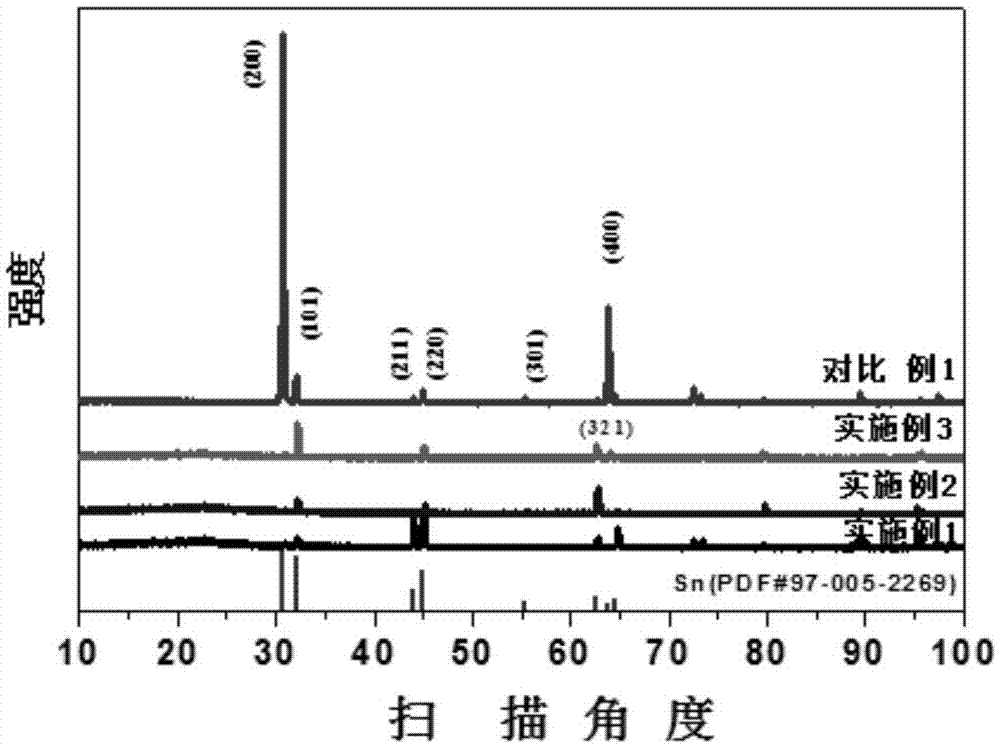
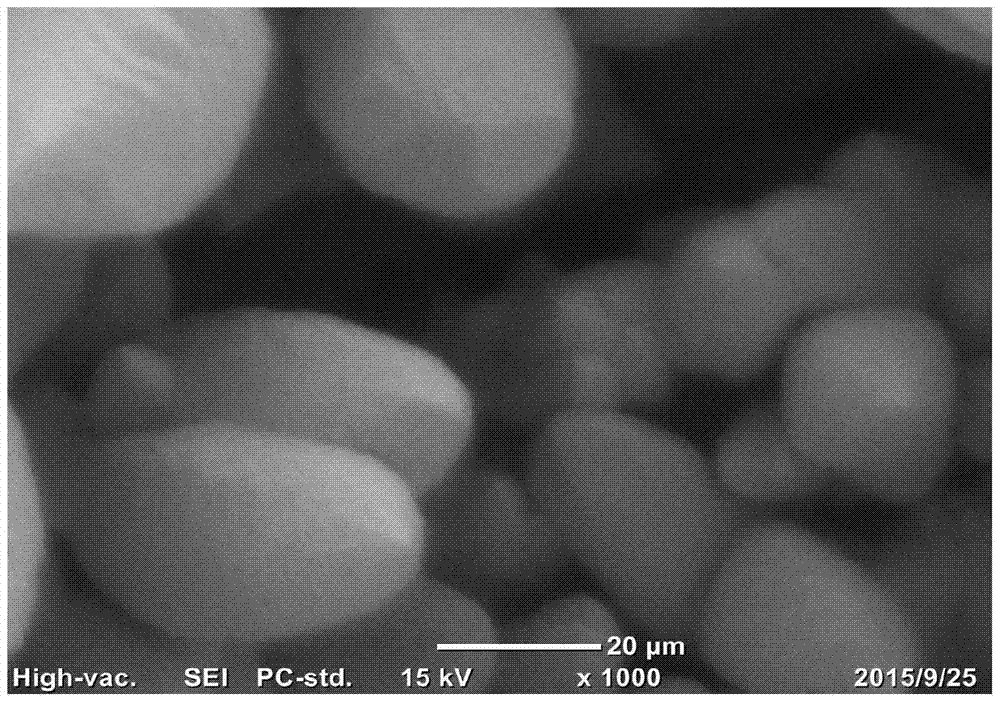
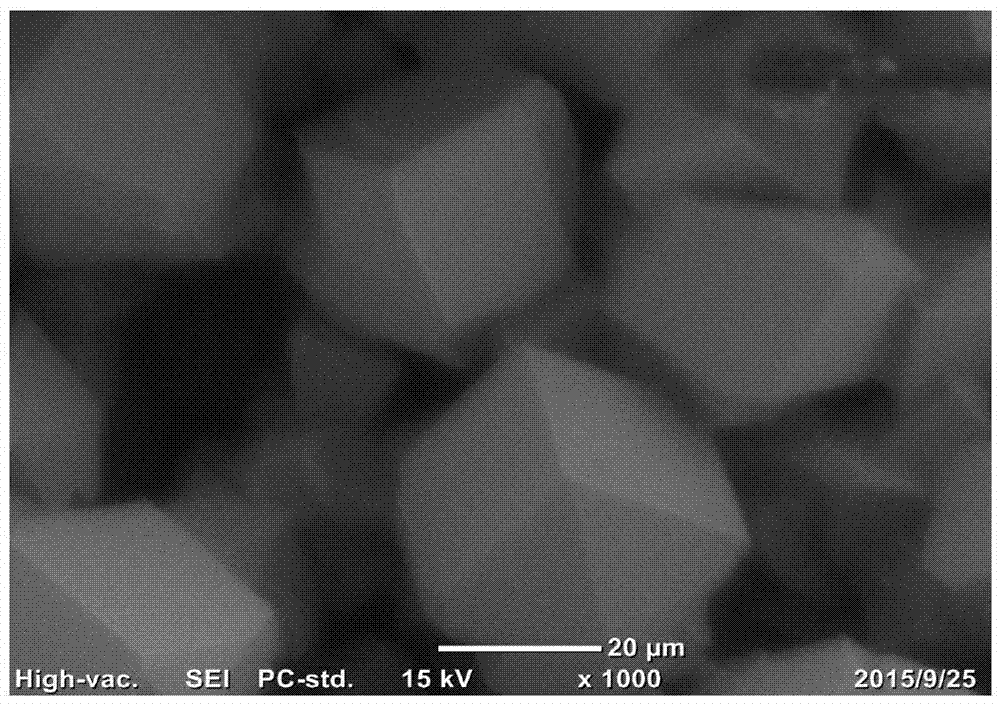
[0038] Treat the carbon paper in the air at 550°C, then degrease in ethanol and acetone and clean it as the substrate; set the concentration to 0.25M SnSO 4 Solution, with 0.7M H 2 SO 4 And 0.06M Trix100, mix evenly to obtain the mixture solution as the electrolyte solution; in the electrolyte solution, in the N 2 Constant current density -60mA cm under atmosphere protection -2 Constant potential deposition for 2000s; 0.5M NaHCO in the post-treatment electrolyte 3 Reduced with -1.2V in the electrolyte solution for 900s; washed and dried to prepare the Sn nanorod catalyst. From Figure 5 It shows that compared with Comparative Example 1, the electrode performance in Example 1 is significantly improved.
Embodiment 2
[0040] Treat the carbon paper in the air at 550°C, then degrease in ethanol and acetone and clean it as the substrate; set the concentration to 0.25M SnSO 4 Solution, with 0.8M H 2 SO 4 And 0.06 2M OP, mix evenly to obtain the mixture solution as the electrolyte solution; in the electrolyte solution, in the N 2 Constant current density -60mA cm under atmosphere protection -2 Constant potential deposition for 2000s; 0.5M NaHCO in the post-treatment electrolyte 3 Reduced with -1.2V in the electrolyte solution for 900s; washed and dried to prepare the Sn nanorod catalyst.
Embodiment 3
[0042] Treat the carbon paper in the air at 550°C, then degrease in ethanol and acetone and clean it as the substrate; set the concentration to 0.25M SnSO 4 Solution, with 0.8M H 2 SO 4 And 0.06M gelatin, mix evenly to obtain the mixture solution as the electrolyte solution; in the electrolyte solution, in N 2 Constant current density -60mA cm under atmosphere protection -2 Constant potential deposition for 2000s; 0.5M NaHCO in the post-treatment electrolyte 3 Reduced with -1.2V in the electrolyte solution for 900s; washed and dried to prepare the Sn nanorod catalyst.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com