Alloy nanoparticles, preparation method and applications thereof
A technology of alloy nanoparticles and metals, which is applied in the field of nanomaterials, can solve the problems of high catalyst cost, cumbersome processing work, complicated process, etc., and achieve good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
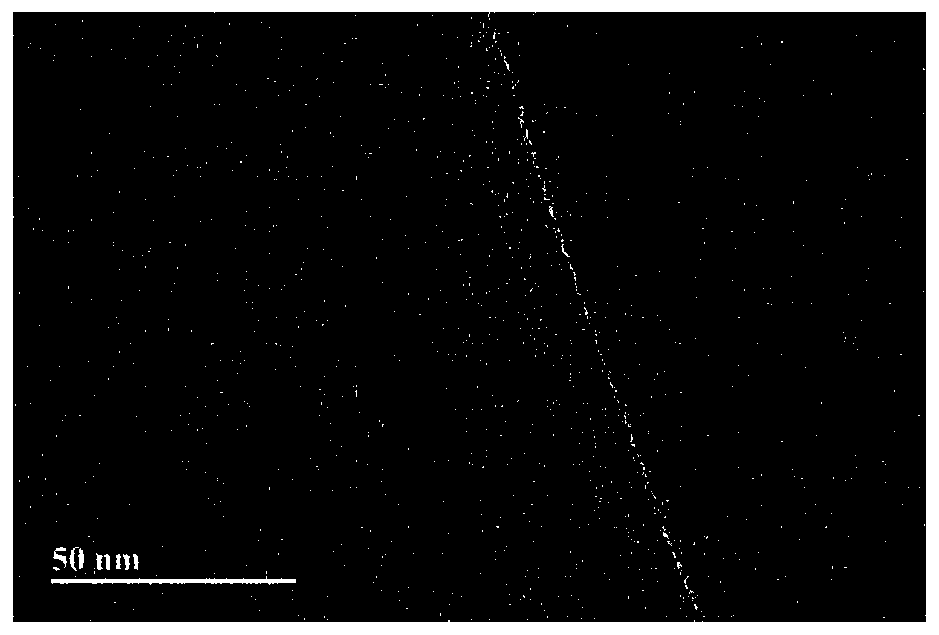
[0048] Add 20 mg of catalyst substrate (graphene oxide) into 20 mL of ultrapure water, and disperse for 8 hours with an ultrasonic cleaning instrument to obtain a uniform and stable catalyst substrate dispersion (1 mg / mL), which is stored in a refrigerator for later use. figure 1 is the topography of a blank graphene oxide catalyst substrate obtained by transmission electron microscopy. Depend on figure 1 It can be seen that the surface of the blank graphene oxide catalyst substrate is smooth and free of particles.
[0049] Prepare a surfactant solution, the volume of the solution is 5mL, the amount of surfactant (tannic acid) is 15mg, and the ultrasound is over 0.5h.
[0050] Configure the metal solution, the metal solution consists of CuCl 2 , IrCl 3 、CoCl 2 , SnCl 2 , PdCl 2 、NiCl 2 、H 2 PtCl 6 , HAuCl 4 , MnCl 2 , RhCl 3 Several compositions of isometallic salts, wherein the total metal concentration is 2mM, and the metals are in equimolar ratio. Ultrasound ...
Embodiment 2
[0053] Referring to the method described in Example 1, the tannic acid in Example 1 was replaced with glucose, and the rest of the reaction conditions were the same as in Example 1 to prepare precursor 2;
[0054] With reference to the method described in Example 1, the tannic acid in Example 1 was replaced with polyvinylpyrrolidone (PVP), and the remaining reaction conditions were the same as in Example 1 to prepare precursor 3;
[0055] Referring to the method described in Example 1, but no surfactant is added in the step, and the remaining reaction conditions are the same as in Example 1, the precursor 4 is prepared;
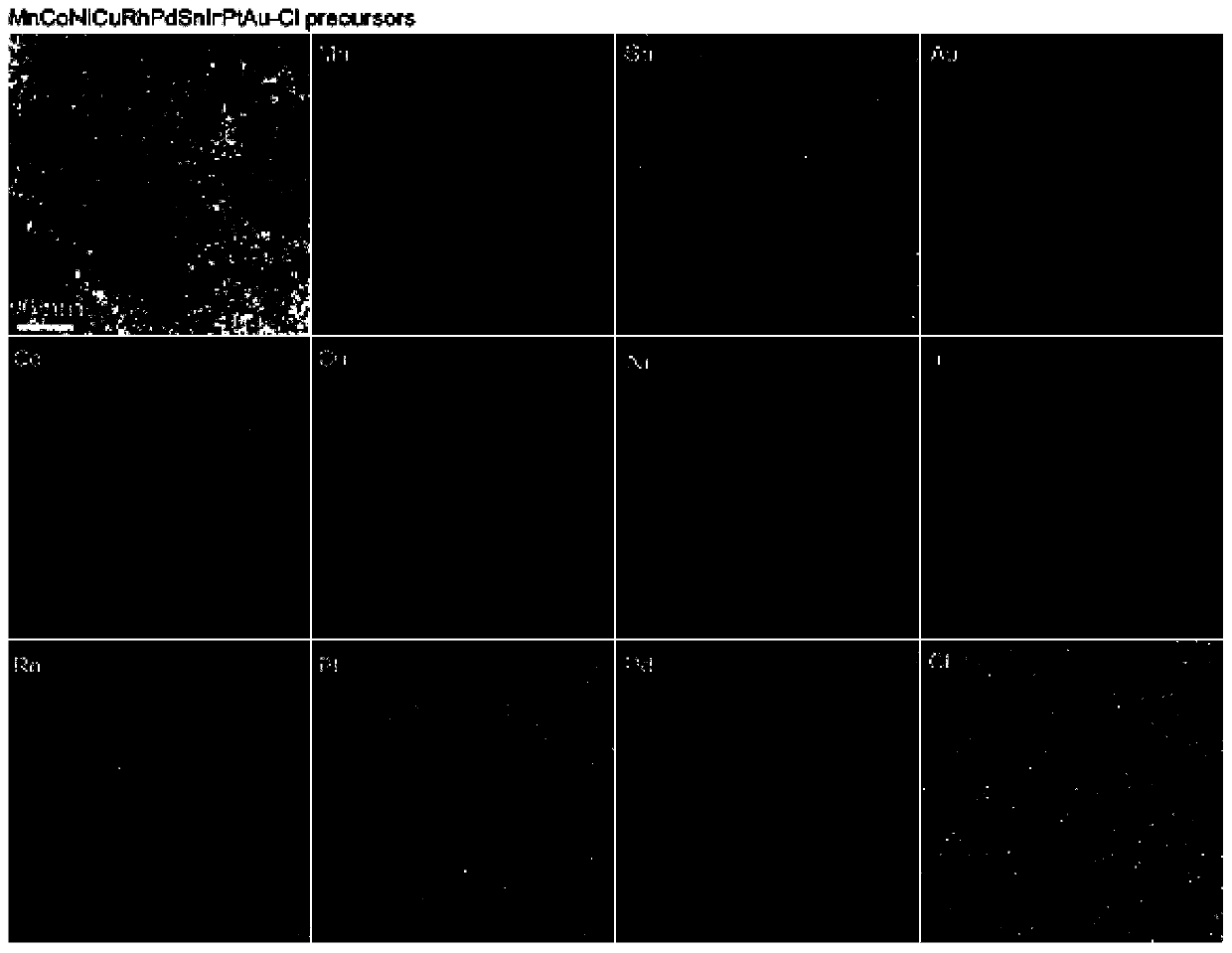
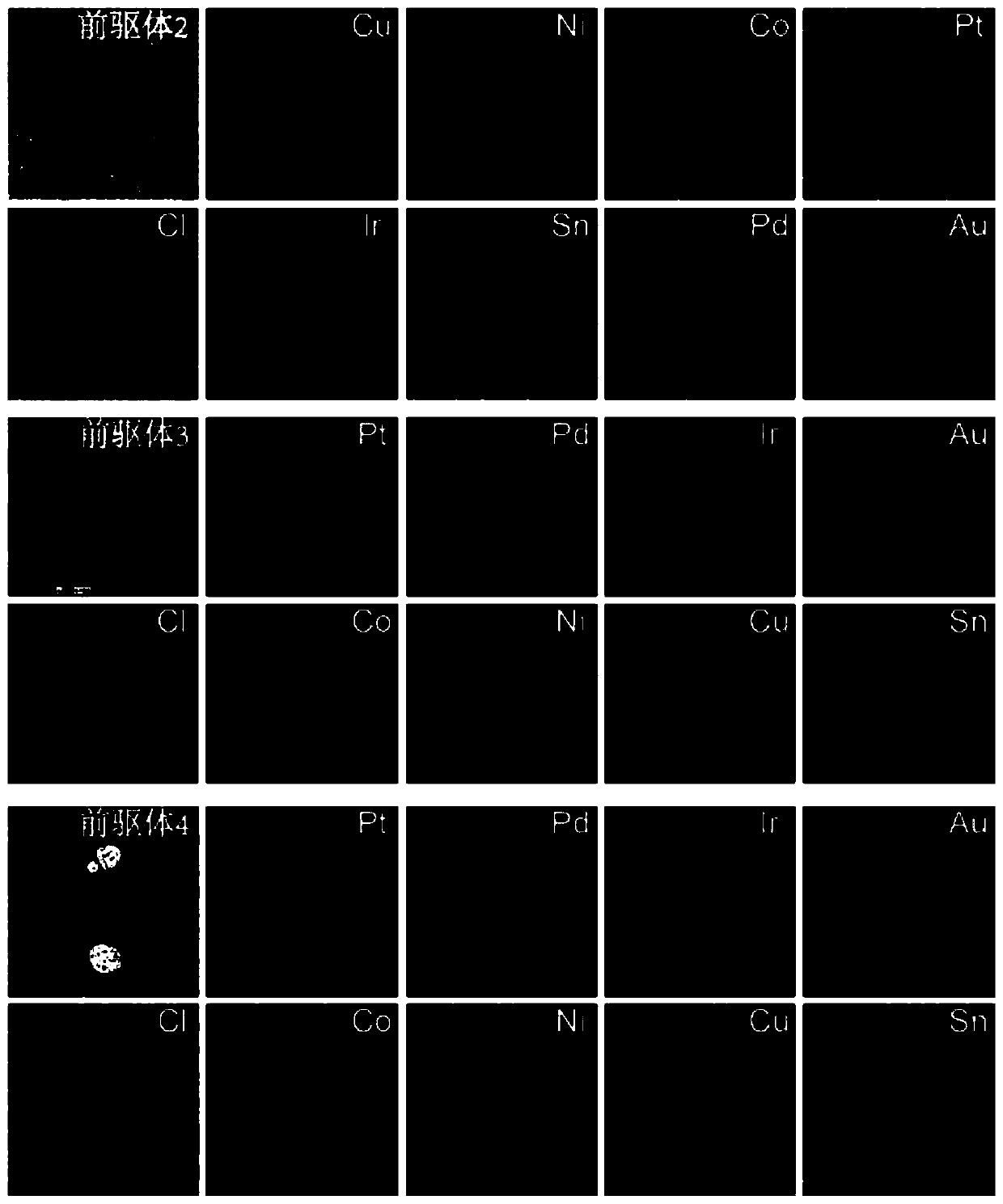
[0056] image 3 It is the morphology map and element distribution map of Precursor 2, Precursor 3 and Precursor 4. Will figure 2 and image 3 For comparison, it can be found that: when the surfactant is tannic acid or glucose, a substance similar to a film is formed on the surface of the catalyst substrate, and various metal ions in the precursor 1 are ev...
Embodiment 3
[0058] The precursor 1 in the embodiment 1 is utilized the fast moving bed pyrolysis method (such as Figure 4 As shown, the precursor 1 is placed in a porcelain boat, and the porcelain boat is placed in the glass tube at the front end of the tube furnace, the inert gas is introduced, the temperature program of the tube furnace is set, the tube furnace is started, and the temperature of the tube furnace is at At 900°C, push the porcelain boat into the tube furnace for rapid temperature rise. After the reaction is completed (1h), the porcelain boat is pushed out of the tube furnace. After the temperature of the sample is lowered (<50° C.), the sample is taken out) to prepare alloy nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com