Thermal activation delayed fluorescence material based on dibenzophenazine derivative as well as preparation method and application of thermal activation delayed fluorescence material
A thermally activated delay, benzophenazine technology, applied in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problem of poor rigid structure, inefficient long-wavelength TADF materials, low fluorescence quantum yield, etc. problem, to achieve the effect of good conjugation ability, excellent electroluminescence performance, and high solid-state fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
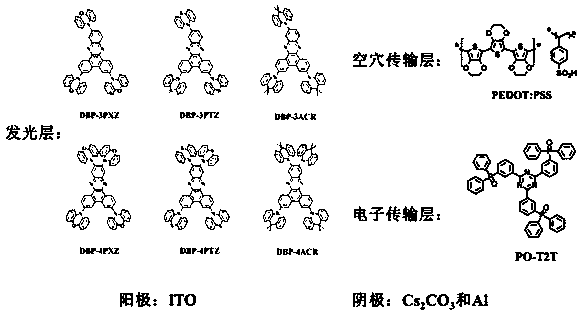
[0041] The thermally activated delayed fluorescent material based on dibenzophenazine derivatives described in this example is DBP-3PXZ, and its structural formula is as follows:
[0042] .
[0043] The preparation method is:
[0044] (1) Add 3,6-dibromophenanthrene-9,10-dione (2 g, 5.46 mmol) into 100 mL of glacial acetic acid and stir to dissolve, then add 4-bromobenzene-1,2-diamine (1.2 g , 6.58 mmol) to react, the reaction temperature is 110 ℃, and the reaction time is 6 hours. After the reaction, the crude product is filtered by suction, washed with water, and dried to obtain the product: 3,6,11-tribromodibenzo[a , c] phenazine, yield 95%.
[0045] (2) Take the product 3,6,11-tribromodibenzo[a,c]phenazine (2 g, 3.87 mmol) and phenoxazine (2.83 g, 15.48 mmol) obtained in step (1) and add Stir in water toluene solution, add tri-tert-butylphosphine (1.16 mL, 11.61 mmol), potassium carbonate (3.2 g, 23.22 mmol) and palladium acetate (0.14 g, 0.6 mmol) to react under nitr...
Embodiment 2
[0057] The thermally activated delayed fluorescent material based on dibenzophenazine derivatives described in this example is DBP-3PTZ, and its structural formula is as follows:
[0058] .
[0059] The preparation method is the same as in Example 1, except that the reactant phenoxazine in step (2) is changed to phenothiazine, and the yield of the reactant is 52%. The molecular structure analysis results are as follows: DBP-3PTZ, mass spectrum: 871.19; elemental analysis: C: 77.13, H: 3.81, N: 8.03.
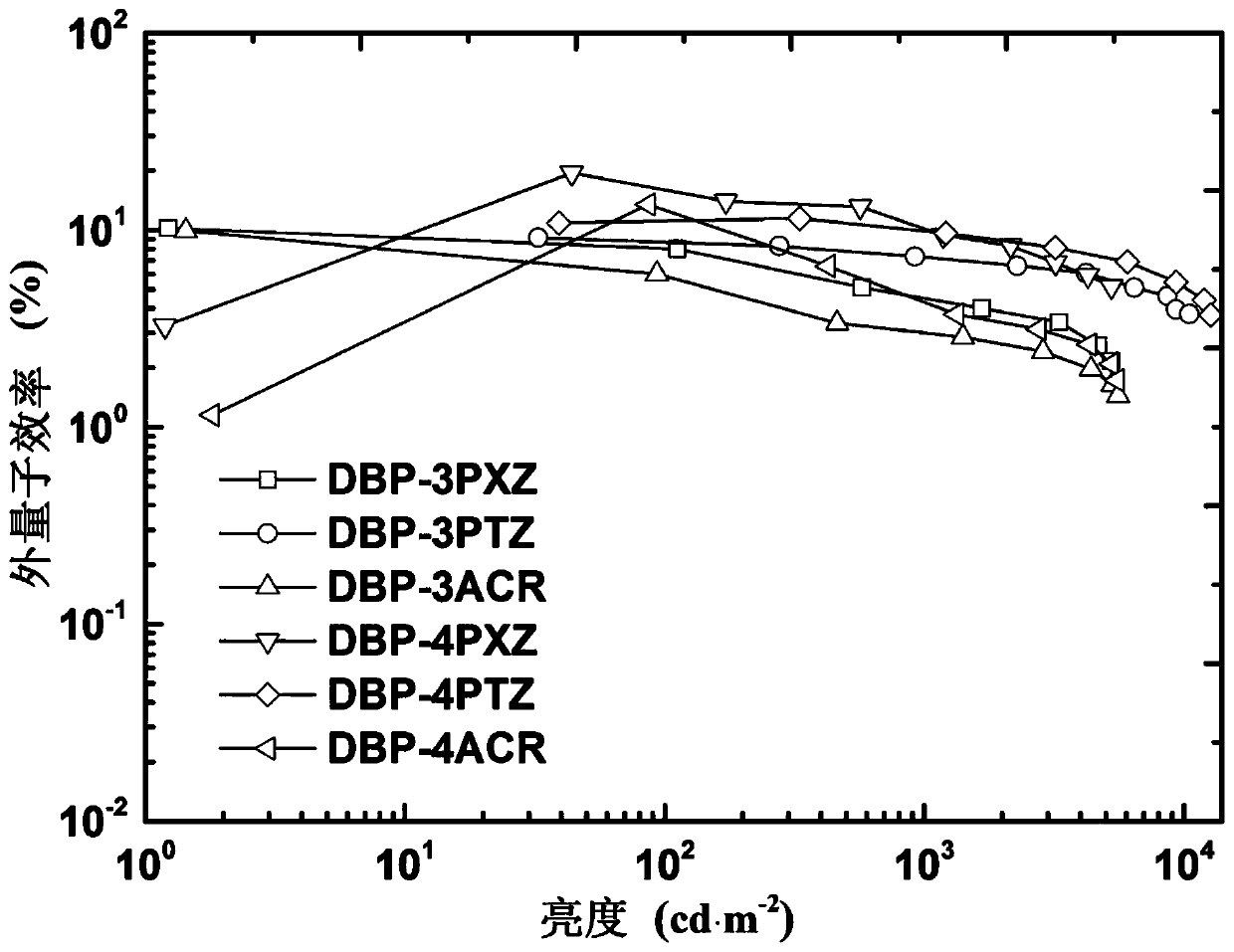
[0060] The application of the above-mentioned DBP-3PTZ thermally activated delayed fluorescent material based on dibenzophenazine derivatives in the preparation of wet-process organic electroluminescent diodes (OLED devices).
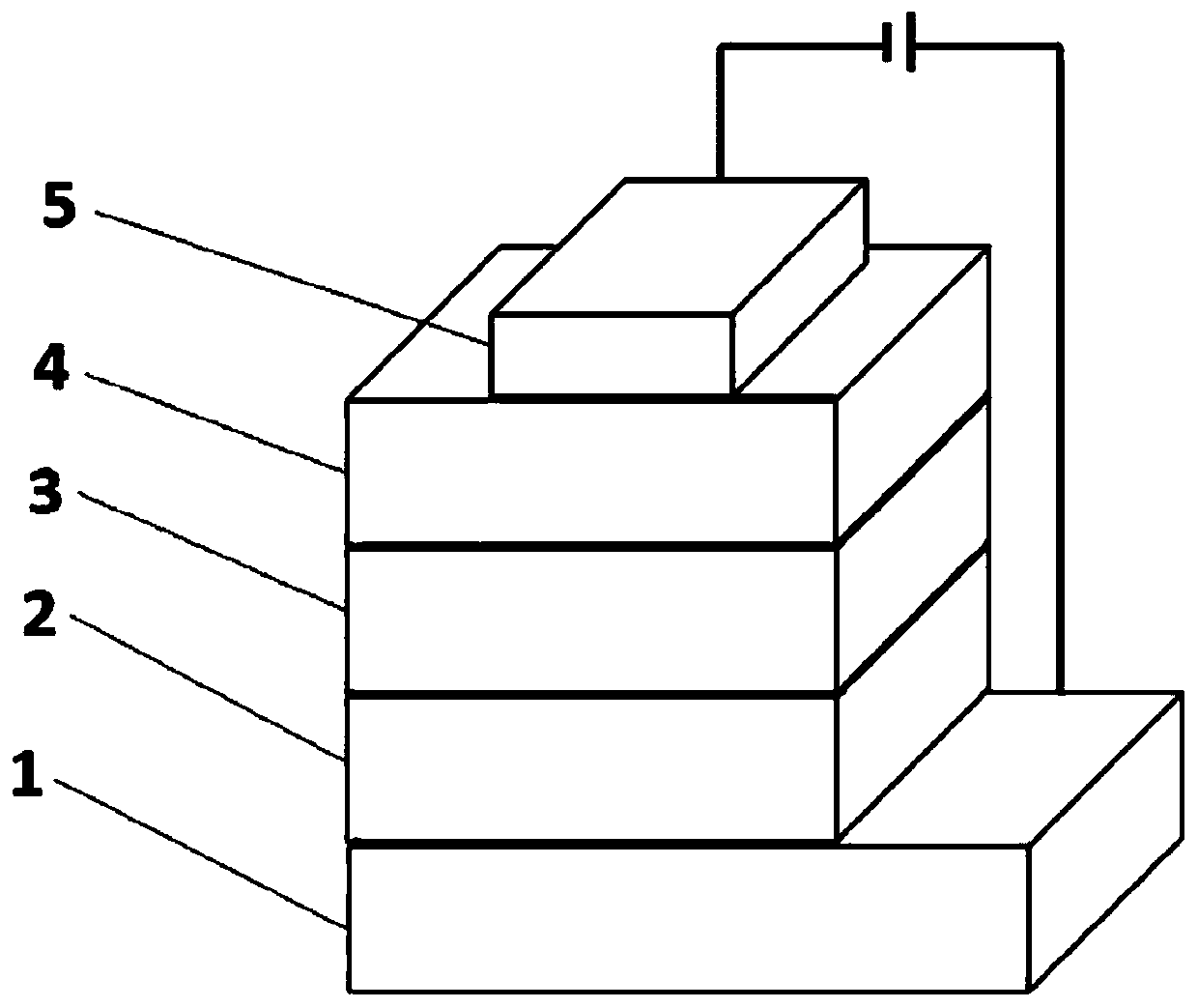
[0061] The schematic diagram of the prepared wet OLED device structure is as follows: from bottom to top, ITO anode 1, hole transport layer 2 (PEDOT:PSS (40 nm)), light emitting layer 3 (DBP-3PTZ (40 nm)), electron transport layer Layer 4 (PO-T2T (40 n...
Embodiment 3
[0065] The thermally activated delayed fluorescent material based on dibenzophenazine derivatives described in this embodiment is DBP-3ACR, and its structural formula is as follows: .
[0066] The preparation method is the same as in Example 1, except that the reactant phenoxazine in step (2) is changed to 9,10-dihydroacridine, and the yield of the reactant is 60%. The molecular structure analysis results are as follows: DBP-3ACR, mass spectrum: 902.16; elemental analysis: C: 86.54, H: 5.70, N: 7.76.
[0067] The application of the above-mentioned DBP-3ACR thermally activated delayed fluorescent material based on dibenzophenazine derivatives in the preparation of wet-process organic electroluminescent diodes (OLED devices).
[0068] The schematic diagram of the prepared wet OLED device structure is as follows: from bottom to top, ITO anode 1, hole transport layer 2 (PEDOT:PSS (40 nm)), light emitting layer 3 (DBP-3ACR (40 nm)), electron transport layer Layer 4 (PO-T2T (40 n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com