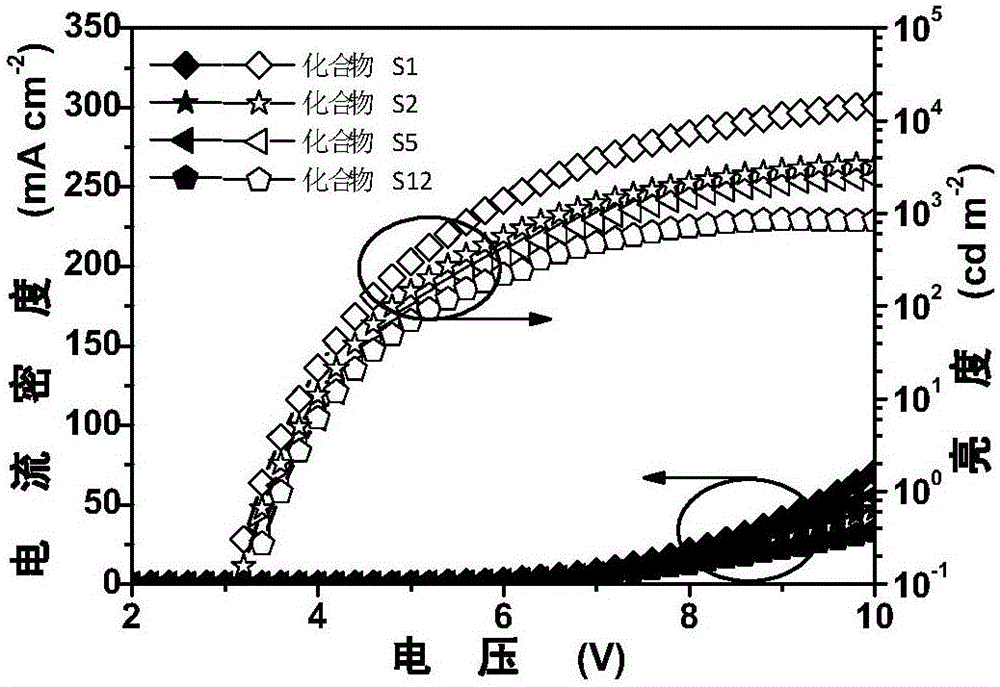
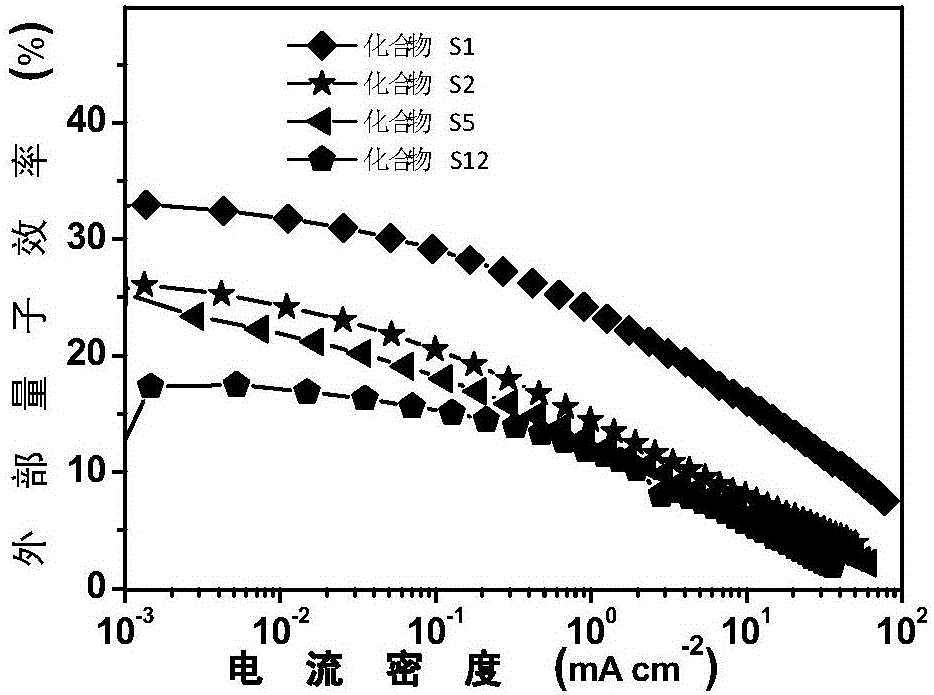
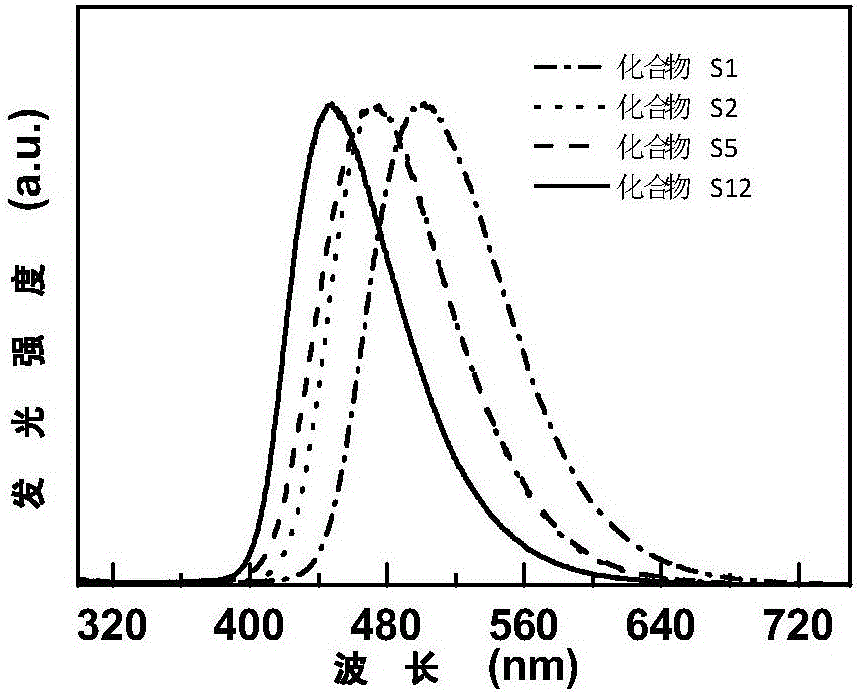
9,9'-spiral acridine-containing compound and preparation method and application thereof
A compound, the technology of spiroacridine, which is applied in the field of compounds containing 9,9'-spiroacridine and its preparation, can solve the problems of low triplet energy level, high degree of molecular conjugation, and difficulty in realizing triplet energy level difference, etc. , achieve high device efficiency, low singlet-triplet energy level difference, and improve the effect of exciton utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] step 1:
[0042] The preparation of (4-bromophenyl)-phenyl-carbamic acid tert-butyl ester, reaction formula is as follows:
[0043]
[0044] In a 1-liter single-necked flask, di-tert-butyl dicarbonate (BOC) (0.2mol, 43.6g) was added to 400ml of tetrahydrofuran at room temperature, and then p-N-(dibromophenyl)aniline (0.1mol , 24.8g), heated to reflux and stirred for 24 hours. The mixture was then poured into 1 L of water, and the product was extracted with dichloromethane. Dry the organic phase with anhydrous magnesium sulfate, remove the solvent after separation, and separate and purify with silica gel chromatography to obtain a colorless oily liquid (32.0 g, yield 92%).
[0045] Step 2:
[0046] The preparation of 10-((2-methoxyethoxy)methyl)-9(10H)acridone, the reaction formula is as follows:
[0047]
[0048]In a 500 ml three-necked flask, 9(10H) acridone (90 mmol, 17.55 g) was added to 200 ml of DMF, and then sodium hydride (120 mmol, about 5.8 g) with a ...
Embodiment 2
[0058] The preparation of compound S2, reaction formula is as follows:
[0059]
[0060] Add 10H,10'H-9,9'-spiroacridine (1.2 mmol, 0.42 g) and 4-(4-bromophenyl)-2,6-diphenylpyrimidine to the reaction flask under argon atmosphere (2.7mmol, 1.04g), 50ml of toluene as solvent, palladium acetate 60mg, tri-tert-butylphosphine (0.5mmol, 0.11g), and 0.48g of sodium tert-butoxide. The reaction was stirred under reflux for 24 hours. After cooling, the mixture was poured into 200 ml of water, and the product was extracted with dichloromethane. Dry the organic phase with anhydrous magnesium sulfate, remove the solvent after separation, and separate and purify with silica gel chromatography to obtain a light yellow solid. After drying, the product was sublimed under vacuum to obtain a high-purity product (0.82 g, yield 71%).
Embodiment 3
[0062] The preparation of compound S3, reaction formula is as follows:
[0063]
[0064] Add 10H,10'H-9,9'-spiroacridine (1.2 mmol, 0.42 g) and 4'-(4-bromophenyl)-2,2';6' to the reaction flask under argon atmosphere , 2 "-terpyridine (2.8mmol, 1.08g), 50 milliliters of toluene as solvent, 60 milligrams of palladium acetate, tri-tert-butylphosphine (0.5 mmol, 0.11 g), and 0.48 gram of sodium tert-butoxide. Stir under reflux Reacted for 24 hours, after cooling, the mixed solution was poured into 200 milliliters of water, and product was extracted with dichloromethane. Anhydrous magnesium sulfate dried the organic phase, separated and removed the solvent, separated and purified with silica gel chromatography to obtain a light-colored solid. After drying, the Sublimation under vacuum gave a high-purity product (0.68 g, yield 59%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com