Thermal activation delayed fluorescent material and organic electroluminescent device
A technology of thermally activated delay and fluorescent materials, which is applied in luminescent materials, electrical solid devices, organic chemistry, etc., can solve the problems of low efficiency of the luminescent layer, achieve high radiative transition rate, small dihedral angle, improve efficiency and stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
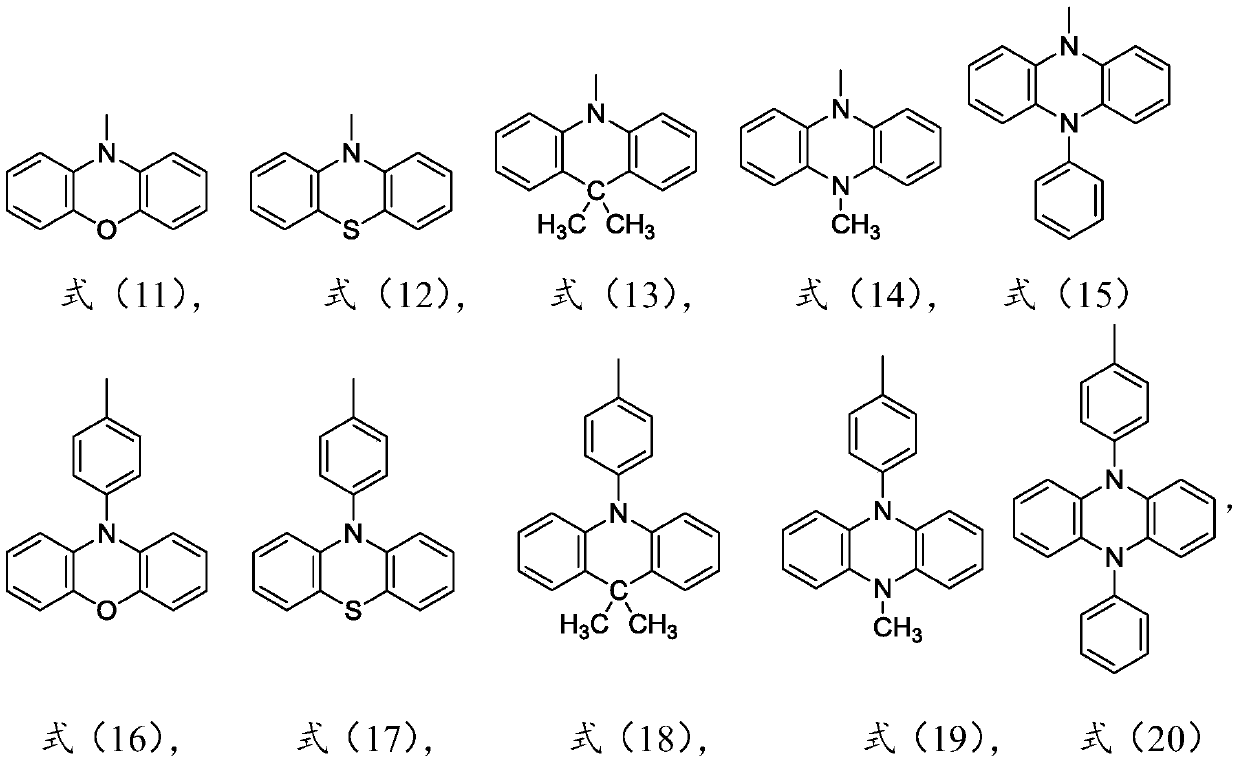
[0068] Synthetic method of the structural compound shown in formula (1-1): under nitrogen range, 3,6-dibromo-9-fluorenone (5mmol), phenoxazine (18mmol), Pd 2 (dba) 3 (0.8mmol), NaOtBu (30mmol) and tBu3P·HBF4 (0.8mmol) were put into 100mL toluene and stirred at 105°C overnight. The reaction was quenched by adding 10 mL of cold water to the mixture. After the mixture was cooled to room temperature, it was vacuum filtered and then purified by column chromatography to obtain the product with the structure shown in formula (1-1). The product was dried in vacuum, yield: 80%.
[0069] The molecular weight obtained by mass spectrometry: 542.58.
[0070] The relative molecular mass percentages of each element obtained by elemental analysis: C: 81.90%; H: 4.09%; N: 5.16%; O: 8.85%.
Embodiment 2
[0072] Synthetic method of the structural compound shown in formula (1-2): the reactant phenoxazine is replaced by phenothiazine, through the same synthetic method as in Example 1, the structural compound shown in formula (1-2) is obtained, and the yield is 91% .
[0073] The molecular weight obtained by mass spectrometry: 574.71.
[0074] The relative molecular mass percentages of each element obtained by elemental analysis: C: 77.32%; H: 3.86%; N: 4.87%; O: 2.78%; S: 11.16%.
Embodiment 3
[0076] Synthesis method of the compound with the structure shown in formula (1-3): the reactant phenoxazine is replaced by 9,9-dimethylacridine, and the structure shown in formula (1-3) is obtained through the same synthesis method as in Example 1 Compound, yield 87%.
[0077] The molecular weight obtained by mass spectrometry: 594.74.
[0078] The relative molecular mass percentages of each element obtained by elemental analysis: C: 86.84%; H: 5.76%; N: 4.71%; O: 2.69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com