N-benzyl-acridone, derivatives of N-benzyl-acridone and preparation methods and application of N-benzyl-acridone and derivatives
A group and reaction technology, applied in the field of N-benzyl-acridone and its derivatives, can solve problems such as hindering the movement of replicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
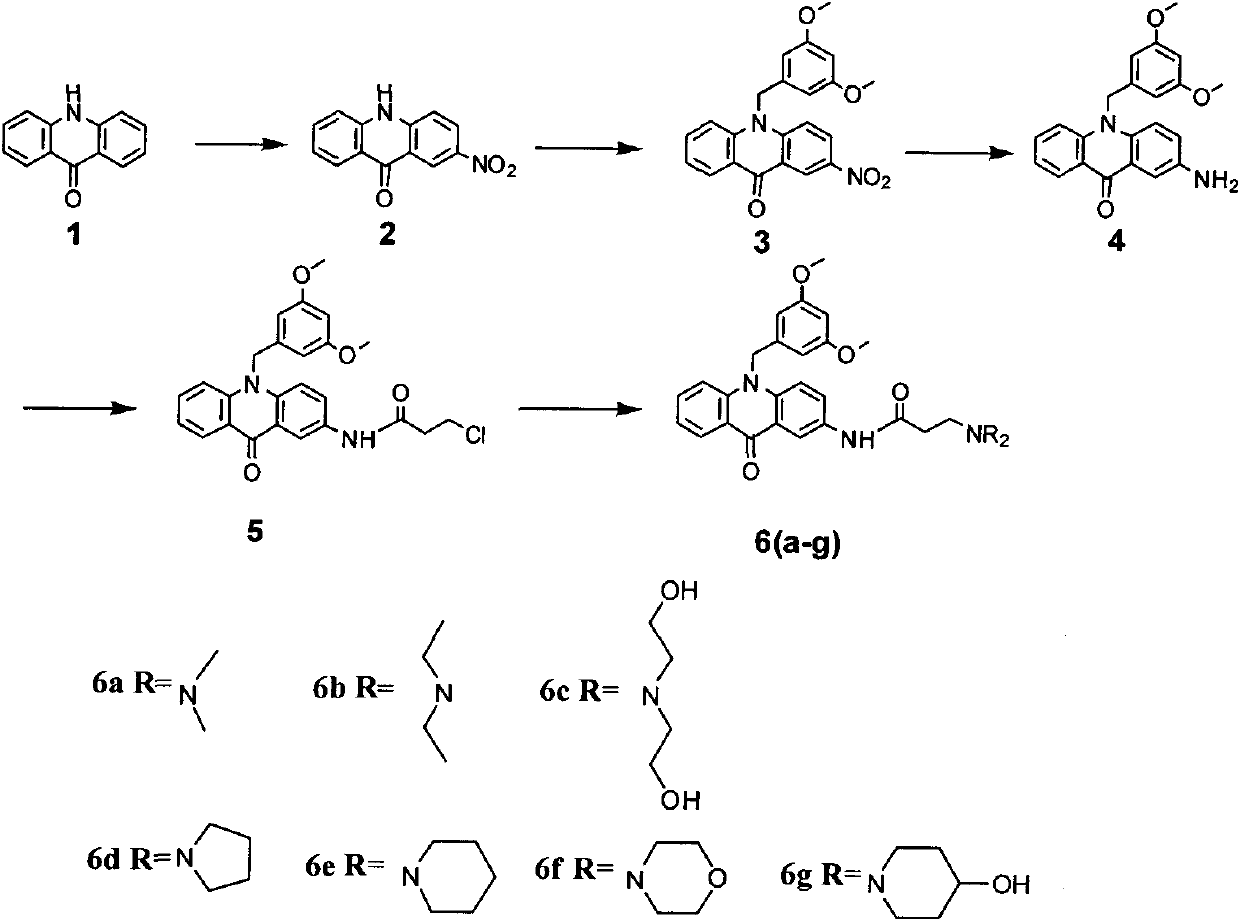
[0066] The preparation of embodiment 1,2-nitro-acridone
[0067] Acridone (1.17g, 6mM) (purchased in Guangzhou Qihua Medical Equipment Co., Ltd., No. A0133), adding 6 ml of acetic acid with a mass concentration of 36%, stirred at room temperature, added 12 ml of glacial acetic acid, 6 ml of concentrated nitric acid (mass The concentration is 65%), keep the temperature at 50-60°C for 4 hours, cool to room temperature, add ice water, precipitate a yellow solid, filter with suction, wash with ice water, recrystallize with ethanol to obtain 1.38 g of product, and directly proceed to the next step reaction , yield 96%.
Embodiment 2
[0068] The preparation of embodiment 2,9-(3,5-dimethoxybenzyl)-2-nitroacridone
[0069] Under the protection of argon, in a three-necked round-bottomed flask equipped with 15 mL of dry DMF, add 720 mg (3 mmol) of 2-nitro-acridone compound, 160 mg (4.0 mmol) of sodium hydride, stir at room temperature for 1 h, and then add 6 mmol of 3, 5-Dimethoxybenzyl chloride, 99mg (0.6mmol) potassium iodide, stirred overnight, added water, stirred rapidly under ice-water bath, yellow crystals precipitated, filtered to obtain a yellow solid, recrystallized with chloroform to obtain 790mg yellow crystals, filtrate After spin-drying and analyzing by column chromatography, 99 mg of the product was obtained, and the yield was 76%. m.p.242-244°C.
[0070] The structural confirmation data are as follows:
[0071] 1 H NMR (300MHz, DMSO-d6) δ: 3.67 (s, 6H), 5.79 (s, 2H), 6.33 (brs, 2H), 6.44 (brs, 1H), 7.47 (m, 1H), 7.70 (m, 1H), 7.79-7.88(m, 2H), 8.38(m, 1H), 8.50(m, 1H), 9.07(m, 1H).
Embodiment 3
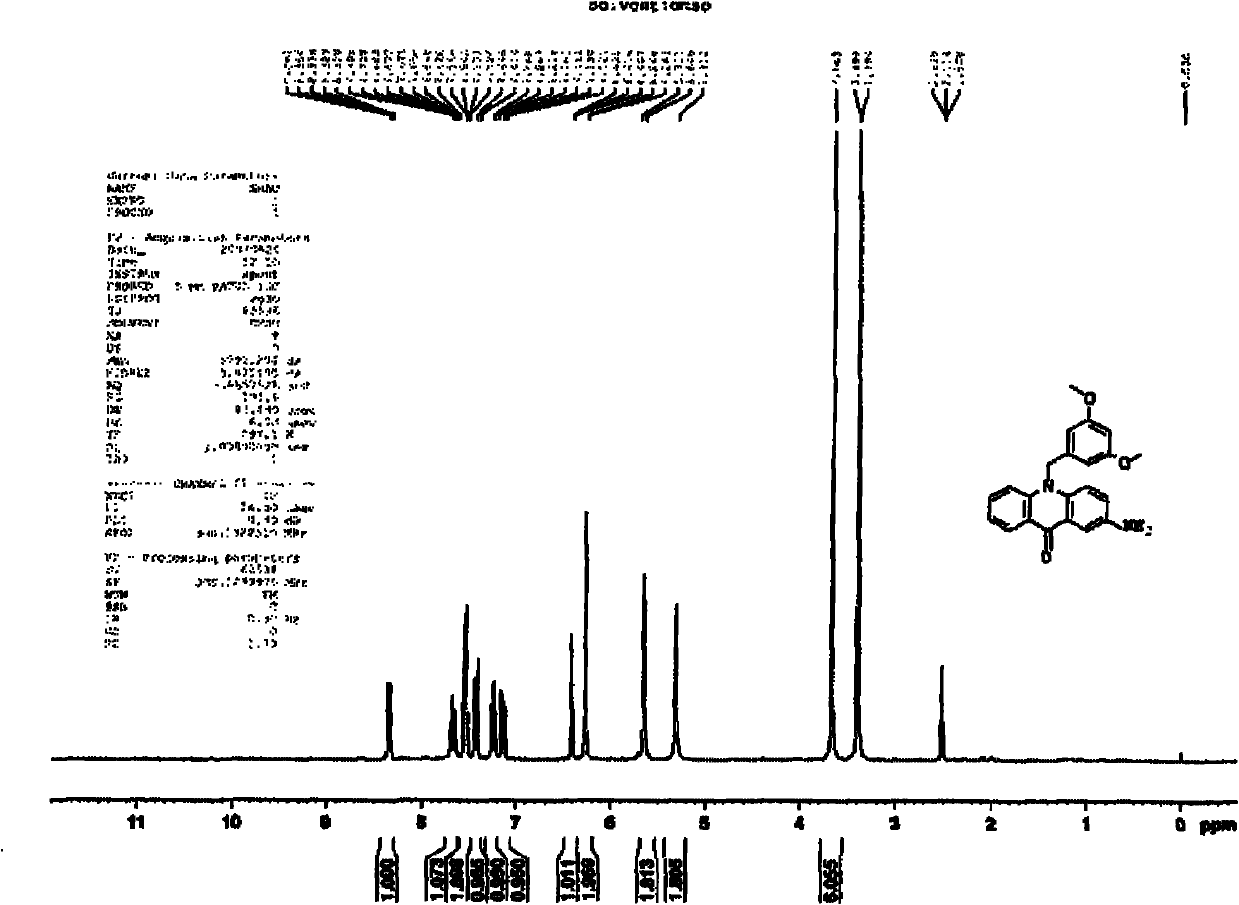
[0072] The preparation of embodiment 3,9-(3,5-dimethoxybenzyl)-2-aminoacridone
[0073] Under argon protection, in a 50ml three-necked round-bottomed flask, add 780mg (2mmol) 9-(3,5-dimethoxybenzyl)-2-nitroacridone compound, 10ml ethanol, 5ml 30% sulfurized Sodium aqueous solution, stirred overnight at about 100°C, added water, stirred rapidly under an ice-water bath, yellow crystals precipitated, filtered to obtain 670 mg of yellow crystals by recrystallization from ethanol as a yellow solid, with a yield of 93%. m.p.290-291°C.
[0074] The structural confirmation data are as follows:
[0075] 1 H NMR (300MHz, DMSO-d6) δ: 3.66(s, 6H), 5.31(s, 2H), 5.65(s, 2H), 6.27(m, 2H), 6.42(m, 1H), 7.14(m, 1H), 7.25(m, 1H), 7.43(m, 1H), 7.53~7.55(m, 2H), 7.67(m, 1H), 8.34(m, 1H);
[0076] 13 C NMR (75MHz, DMSO-d6) δ: 49.4, 55.6, 98.7, 104.6, 107.7, 116.2, 117.6, 120.9, 121.0, 123.5, 123.9, 127.2, 134.0, 134.5, 139.8, 141.9, 144.3, 161.4, 176.6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com