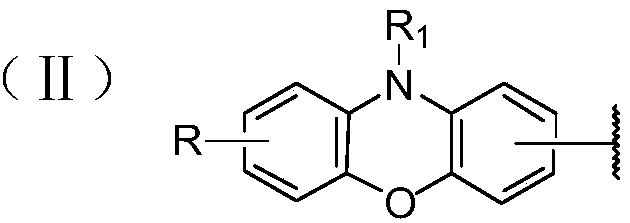
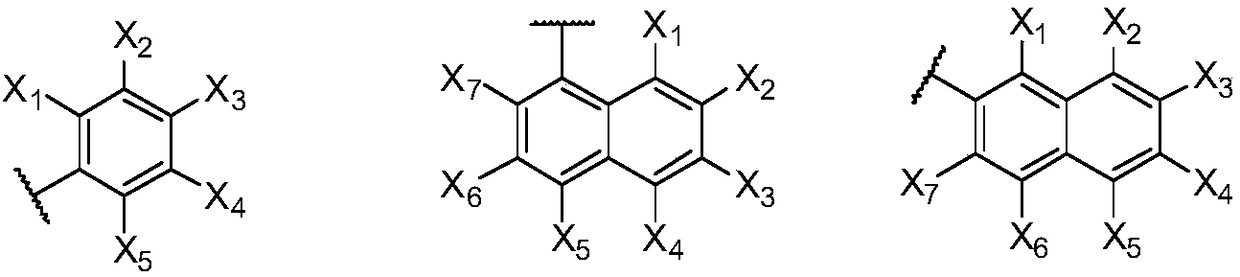
Phenoxazine derivative and organic light-emitting device thereof
A technology of phenoxazine derivatives and electroluminescent devices, which is applied in the field of organic photoelectric materials, and can solve the problems of short life of organic light-emitting devices, reduced material usage, poor thermal stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Preparation of compound 1
[0076]
[0077] Preparation of Compound 1-3
[0078] Compounds 1-1 (5.16 g, 20 mmol), 1-2 (4.28 g, 20 mmol) and sodium ethoxide were completely dissolved in 100 ml of ethanol in a round bottom flask, and the resulting solution was heated and stirred. After the reaction was completed, the residue obtained by concentrating the obtained product under reduced pressure was diluted with tetrahydrofuran, and washed with water and brine. The organic solvent layer was collected, water was removed over anhydrous magnesium sulfate, and the residue was filtered, then concentrated under reduced pressure. The concentrated solution was purified by silica gel column chromatography to prepare Compound 1-36.81 g in a yield of 75%.
[0079] Preparation of compound 1
[0080] Under the protection of nitrogen, 1-3 (45.38g, 100mmol), 1-4 (38.52g, 100.08mmol), potassium carbonate (1.24g, 9.00mmol), and toluene 200mL were added to a 2L reactor and stirred. Th...
Embodiment 2
[0082] Preparation of compound 16
[0083]
[0084] Preparation of compound 16
[0085] Compound 16 was obtained by replacing 1-2 in Example 1 with 16-2 shown above, and 1-4 with 16-4 shown above. Mass Spectrum m / z: 512.16 (calculated: 512.58). Theoretical element content (%)C 35 h 24 N 4 O: C, 82.01; H, 3.93; N, 10.93; O, 3.12 The measured element content (%): C, 82.02; H, 3.92; N, 10.94; O, 3.13. It was confirmed above that the obtained product was the target product 16.
Embodiment 3
[0087] Preparation of compound 27
[0088]
[0089] Preparation of compound 27
[0090]Compound 27 is obtained by replacing 1-2 in Example 1 with 27-2 shown above, and 1-4 with 27-4 shown above. Mass Spectrum m / z: 575.16 (calculated: 575.17). Theoretical element content (%)C 40 h 21 N 3 o 2 : C, 83.46; H, 3.68; N, 7.30; O, 5.56 The measured element content (%): C, 83.45; H, 3.67; N, 7.31; O, 5.55. It was confirmed above that the obtained product was the target product 27.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com