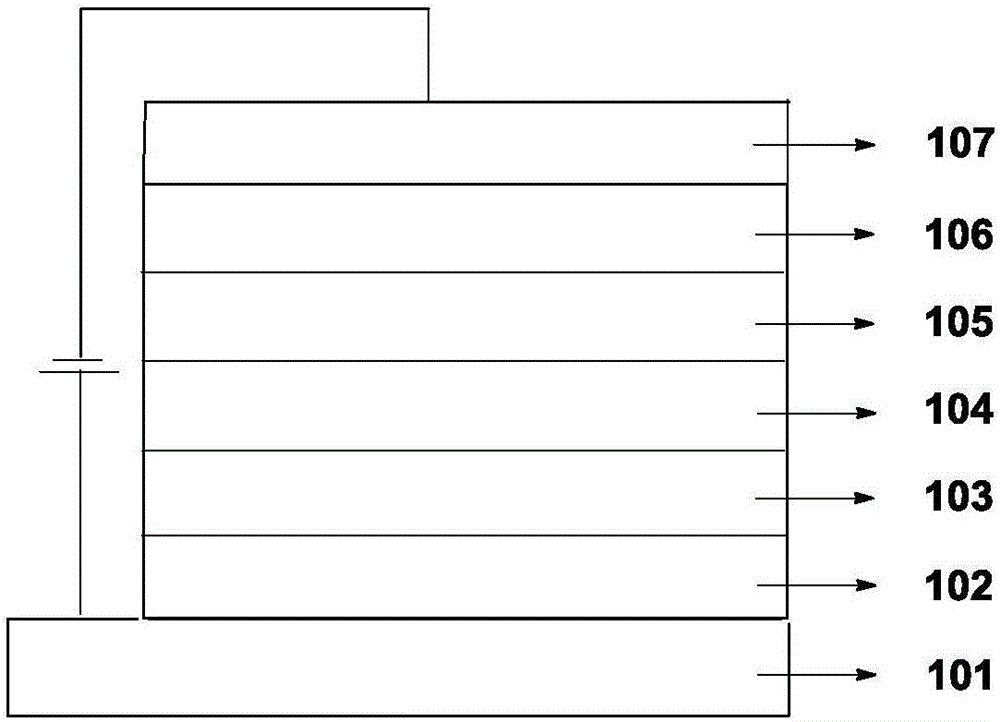
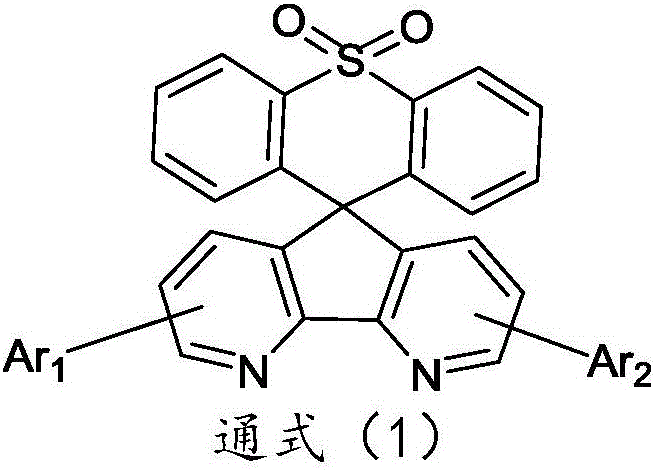
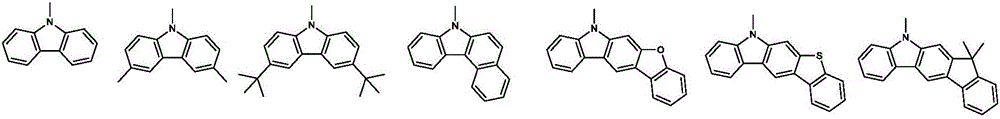
Organic electroluminescence material comprising 4,5-diazaspiro thioxanthone structure, application of organic electroluminescence material, and device
An electroluminescent material, diazaspiro technology, applied in luminescent materials, electric solid devices, electrical components, etc., can solve the problems of unbalanced OLED turn-on voltage and life, single carrier transport properties, high turn-on voltage, etc. , to achieve the effect of improving external quantum efficiency, high glass transition temperature and thermal decomposition temperature, and low turn-on voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of intermediate M1
[0036]
[0037] Under nitrogen protection, diphenylsulfone (10.9 g, 0.05 mol) and tetrahydrofuran (109 mL) were added into a 500 mL three-necked flask. The bath temperature was set at -85°C, and the temperature was lowered with stirring. When the internal temperature dropped to -80°C, a solution of n-butyllithium in n-hexane (24 mL, 2.5M) was added dropwise, and the dropping rate was controlled to keep the internal temperature below -80°C. After the dropwise addition, keep the reaction for 3h, move to room temperature at 25°C and stir for 2h. A solution of methyl 5-bromo-[2,2'-bipyridine]-3-carboxylate (14.6 g, 0.05 mol) in tetrahydrofuran (100 mL) was added dropwise. After the dropwise addition was completed, the mixture was stirred for 10 h at room temperature at 25°C. 100mL of saturated ammonium chloride aqueous solution was used to quench the reaction, and hydrolyzed to generate corresponding alcohol intermedi...
Embodiment 2
[0041] Embodiment 2: the preparation of intermediate M2
[0042]
[0043] Using a method similar to Example 1, using an equivalent amount of 6-bromo-[2,2'-bipyridine]-3-methyl carboxylate instead of 5-bromo-[2,2'-bipyridine]-3- Methyl formate, with other conditions unchanged, intermediate M2 was obtained, 5.3 g of off-white solid, yield 23%.
[0044] Mass spectrum MS (m / e), molecular formula C 23 h 13 BrN 2 o 2 S, theoretical value 459.9, test value 459.6.
[0045] Elemental analysis (C 23 h 13 BrN 2 o 2 S), theoretical value C: 59.88%, H: 2.84%, Br: 17.32, O: 6.94%, N: 6.07%, S: 6.95%; measured value C: 59.87%, H: 2.79%, Br: 17.40, O: 6.99%, N: 6.10%, S: 6.85%.
Embodiment 3
[0046] Embodiment 3: the preparation of intermediate M3
[0047]
[0048] Using a method similar to Example 1, using an equivalent amount of 5,5'-dibromo-[2,2'-bipyridine]-3-methyl carboxylate instead of 5-bromo-[2,2'-bipyridine ]-3-Methyl carboxylate, other conditions remained unchanged, to obtain intermediate M3, off-white solid 11.3g, yield 42%.
[0049] Mass spectrum MS (m / e), molecular formula C 23 h 12 Br 2 N 2 o 2 S, theoretical value 537.9, test value 538.1.
[0050] Elemental analysis (C 23 h 12 Br 2 N 2 o 2 S), theoretical value C: 51.14%, H: 2.24%, Br: 29.58, O: 5.92%, N: 5.19%, S: 5.94%; measured value C: 51.22%, H: 2.25%, Br: 29.47, O: 5.95%, N: 5.20%, S: 5.91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com