Room temperature phosphorescent compound and composition and application thereof
A room temperature phosphorescence, compound technology, applied in the field of phosphorescence compounds, can solve problems such as inability to achieve electroluminescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Table 1. Summary of Synthesis Example Product Data.
[0092]
[0093]
[0094] 3mmol of raw material-1 (ie 2,6-dibromonaphthalene or its derivatives: N-1 to N-62), 6.6mmol of raw material-2 (ie phenothiazine or its derivatives: PTZ-1 to PTZ -25), 19.8mmol sodium tert-butoxide, 0.15mmol palladium acetate and 0.15mmol tri-tert-butylphosphine were added to 35mL toluene, refluxed for 24 hours under the protection of nitrogen, then cooled to room temperature, the reaction solution was filtered, and added to the filtrate Add 100mL water, extract the organic phase with 100mL dichloromethane, separate the organic phase and concentrate to 5.5mL, carry out column chromatography separation with dichloromethane and petroleum ether (volume ratio 1:3) eluent to obtain the target product, then The pure product was obtained by vacuum sublimation. The relevant data of the target compounds obtained are shown in Table 1.
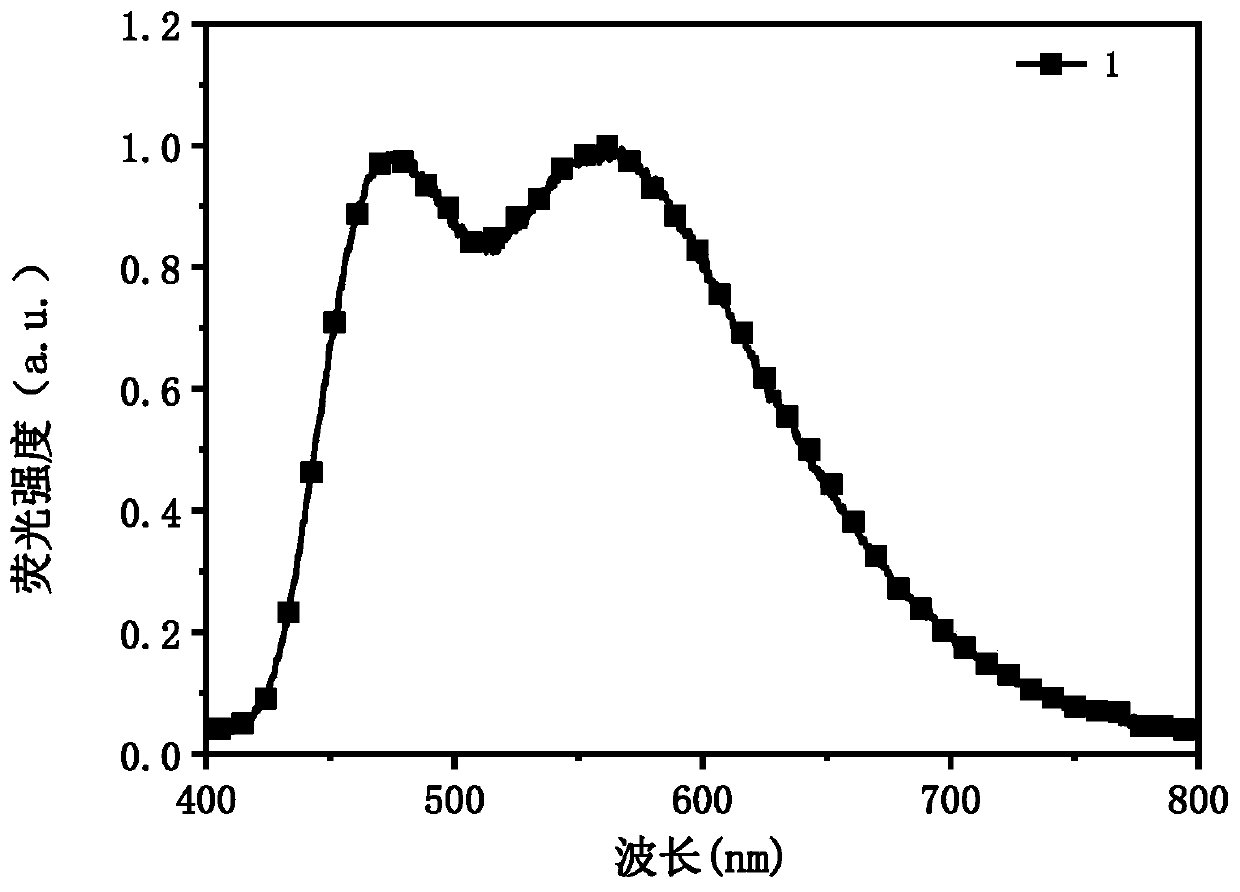
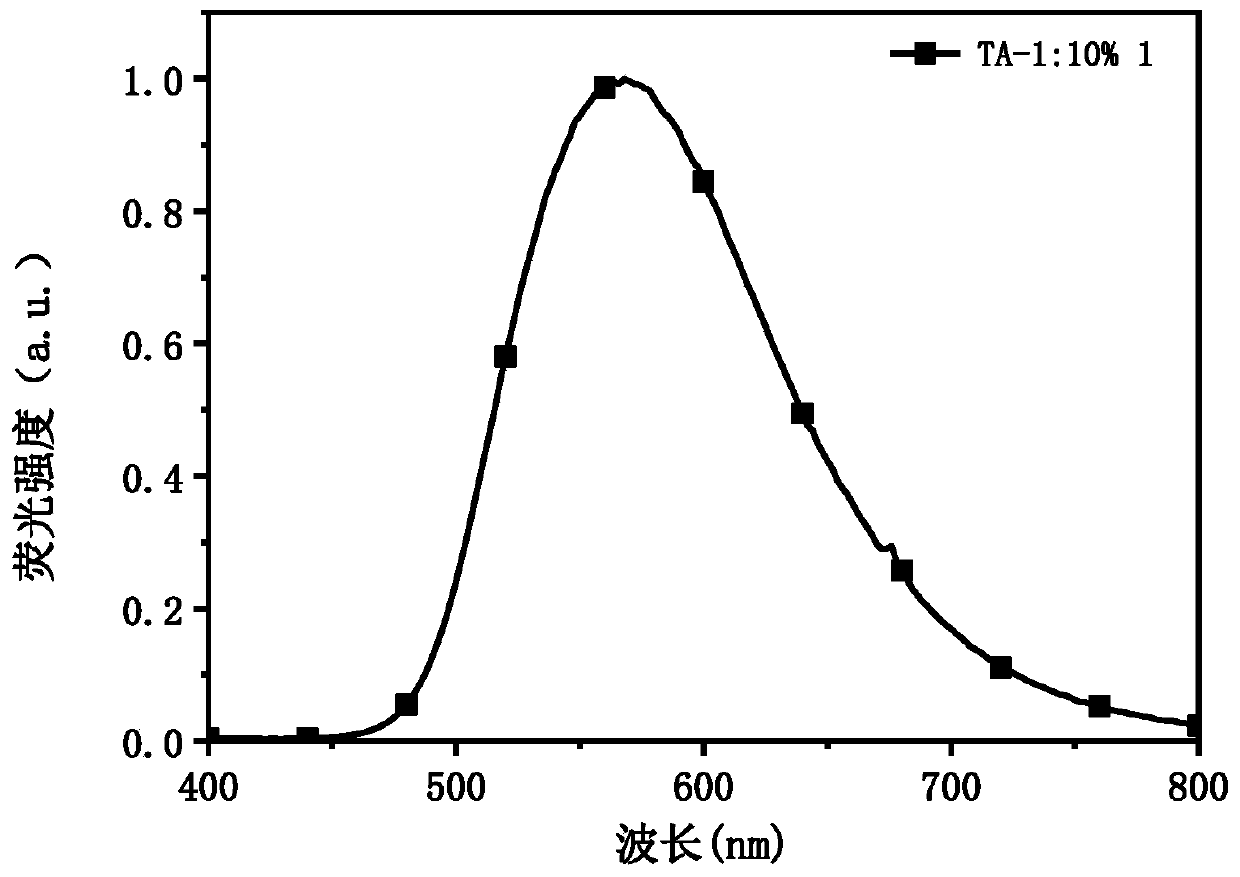
[0095] Taking compound 1 as an example to illustrate the s...
Embodiment 2
[0097] Table 2. Summary of Synthesis Example Product Data.
[0098]
[0099] 3 mmol of 1,4 dibromonaphthalene or its derivatives (raw material-1: N-63 to N-123), 6.6 mmol of phenothiazine or its derivatives (raw material-2: PTZ-1 to PTZ-25), Add 19.8mmol sodium tert-butoxide, 0.15mmol palladium acetate and 0.15mmol tri-tert-butylphosphine to 35mL toluene, reflux for 24 hours under nitrogen protection, then cool to room temperature, filter the reaction solution, add 100mL water to the filtrate, Extract the organic phase with 100mL dichloromethane, separate the organic phase and concentrate to 5.5mL, use dichloromethane and petroleum ether (volume ratio 1:3) eluent to carry out column chromatography separation to obtain the target product, and then obtain the target product by vacuum sublimation Pure. The relevant data of the obtained target compounds are shown in Table 2.
[0100] Taking compound 76 as an example to illustrate the specific details of the synthesis example ...
Embodiment 3
[0102] With 3mmol of raw material-1 (ie 2,6-dibromonaphthalene or its derivatives: N-1 to N-62), 6.6mmol of raw material-2 (ie subthiazine or its derivatives: PXZ-1 to PXZ -25), 19.8mmol cesium carbonate, 0.15mmol bis(dibenzylideneacetone) palladium and 0.15mmol tri-tert-butylphosphine were added to 35mL o-dichlorobenzene, heated to reflux for 24 hours under the protection of nitrogen, and then cooled To room temperature, filter the reaction solution, add 100mL water to the filtrate, extract the organic phase with 100mL dichloromethane, separate the organic phase and concentrate to 5.5mL, elute with dichloromethane and petroleum ether (volume ratio 1:3) Reagents were separated by column chromatography to obtain the target product, and then the pure product was obtained by vacuum sublimation. The relevant data of the obtained target compounds are shown in Table 3.
[0103] Taking compound 123 as an example to illustrate the specific details of the experiment in the synthesis e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com