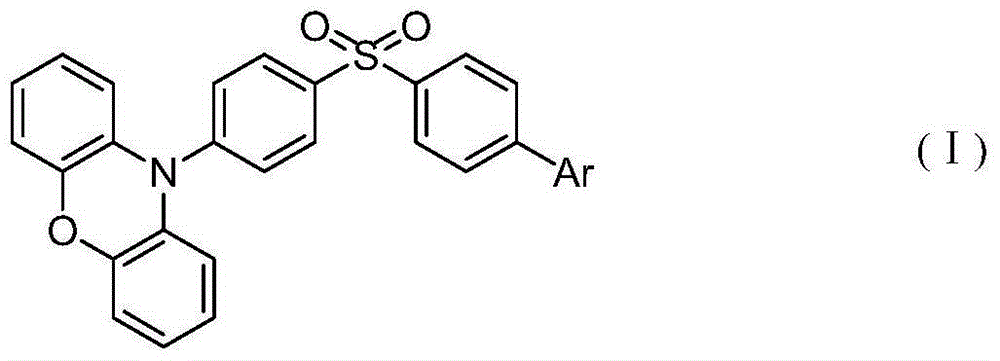
Asymmetrical thermal-activation-delayed aggregation-induced emission material based on diphenyl sulfone phenoxazine, as well as synthesis method and application of material
A technology of aggregation-induced luminescence and thermal activation delay, which can be used in luminescent materials, chemical instruments and methods, semiconductor devices, etc. Effects of simple transition temperature, synthesis method and purification process, and excellent luminescence properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The synthesis method of thermally activated delayed fluorescent material of the present invention comprises the following steps:
[0038] (1) Connect fluorobenzene to 4-iodobenzenesulfonyl through Friedel-Crafts reaction to obtain the first intermediate 1-(4-fluorobenzenesulfonyl)-4-iodobenzene.
[0039] (2) connecting the phenoxazine to the first intermediate prepared in step (1) through a coupling reaction to obtain the second intermediate. Specifically, the coupling reaction for synthesizing the second intermediate is realized by the following method: the first intermediate is reacted with phenoxazine under the action of potassium tert-butoxide to synthesize the second intermediate.
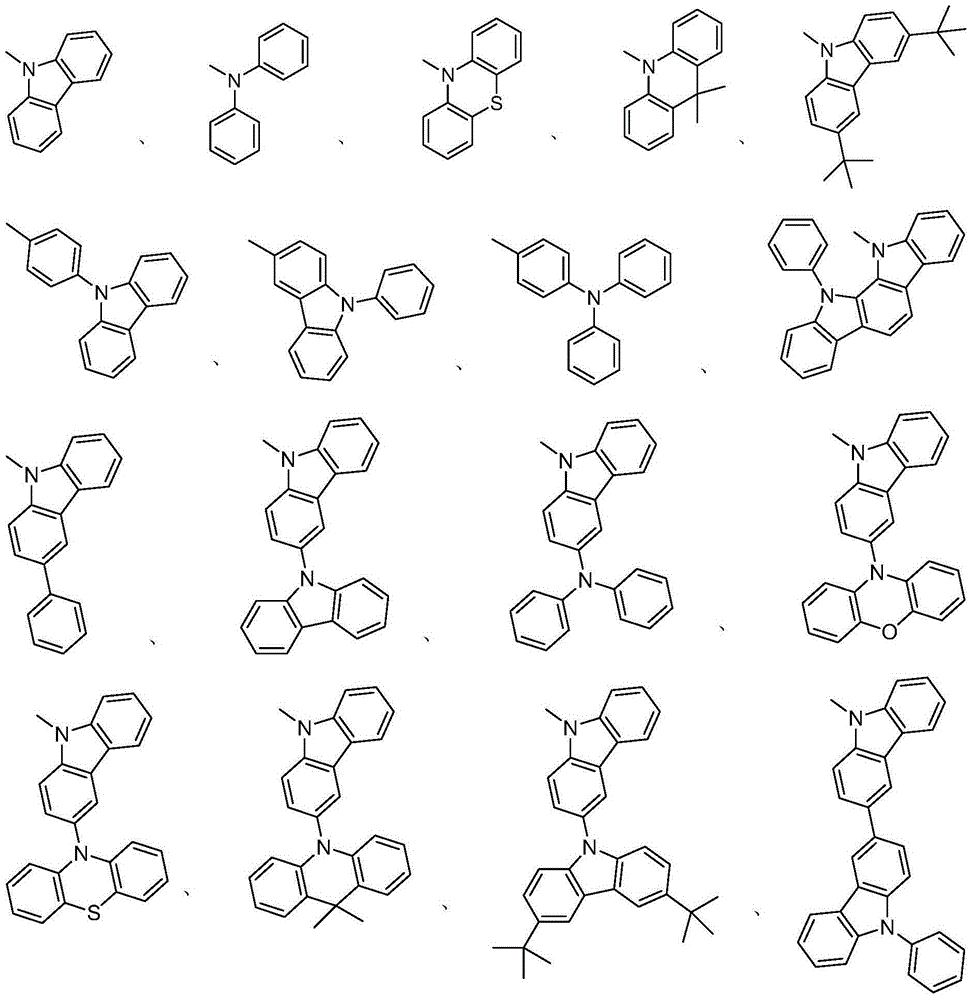
[0040](3) Phenylboronic acid, carbazole, diphenylamine, phenothiazine, phenoxazine, dimethylacridine, 3,6-di-tert-butylcarbazole, triphenylamine borate, 4-(9-carbazolyl ) A kind of in phenylboronic acid or N-phenylcarbazole-3-boronic acid is connected on other carbazoles by coupling re...
Embodiment 1
[0045] Example 1: 10-(4-((4'-(9H-carbazol-9-yl)-[1,1'-biphenyl]-4-yl)sulfonyl)phenyl)-10H-phen Synthesis of oxazines
[0046] (1) Synthesis of the first intermediate 1-(4-fluorobenzenesulfonyl)-4-iodobenzene:
[0047]
[0048] Add 4-iodobenzenesulfonyl chloride (5.91g, 19.53mmol) and fluorobenzene (2.81g, 29.30mmol) into a 250mL three-necked flask, add ferric chloride (7.91g, 48.83mmol) and heat the reaction solution to 40 ° C and stirred the reaction for 3h. The reaction solution was cooled to room temperature, 30 mL of dichloromethane and 50 mL of 1M dilute hydrochloric acid were added successively and stirred for 10 min, the mixed solution was poured into a separatory funnel, the organic layer solution was taken, dried with anhydrous sodium sulfate, and suction filtered, and the obtained filtrate was The solvent was spin-dried by the evaporator, and the remaining solid was vacuum-dried to obtain 6.72 g of yellow-white powder, with a yield of 95%.
[0049] (2) Synthesi...
Embodiment 2
[0055] Example 2: Synthesis of 10-(4-((4-(9H-[3,9'-dicarbazol]-9-yl)phenyl)sulfonyl)phenyl)-10H-phenoxazine
[0056] (1) Synthesis of intermediate 3-bromo-9-tosyl-9H-carbazole:
[0057]
[0058] Add 3-bromocarbazole (5.00g, 20.32mmol), potassium hydroxide (3.41g, 60.95mmol) and 50mL of acetone into a 250mL three-neck flask and stir for 10min, add p-toluenesulfonyl chloride (11.62g, 60.95mmol), and heat to reflux After 1 hour, a large amount of white solid was observed to be precipitated, which was filtered by suction. The solid was washed twice with a small amount of ethanol and dried in vacuo to obtain 7.48 g of white powder with a yield of 92%.
[0059] (2) Synthesis of intermediate 3-(9H-carbazol-9-yl)-9-tosyl-9H-carbazole:
[0060]
[0061] The intermediate 3-bromo-9-tosyl-9H-carbazole (1.00g, 2.50mmol), carbazole (0.63g, 3.75mmol), K 2 CO 3 (1.03g, 7.49mmol), 1,10-phenanthroline (0.10g), 18-crown-6 (0.10g), cuprous iodide (0.20g) and 30mL DMF were added to a 250m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com