Naphthalimide compound with active end and preparation method and application thereof
A naphthalimide and active end technology, which is applied in the field of naphthalimide compounds and their preparation, can solve the problems of lack of targeted modification, and achieve the effects of good luminescent performance in aggregated state, simple synthesis, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
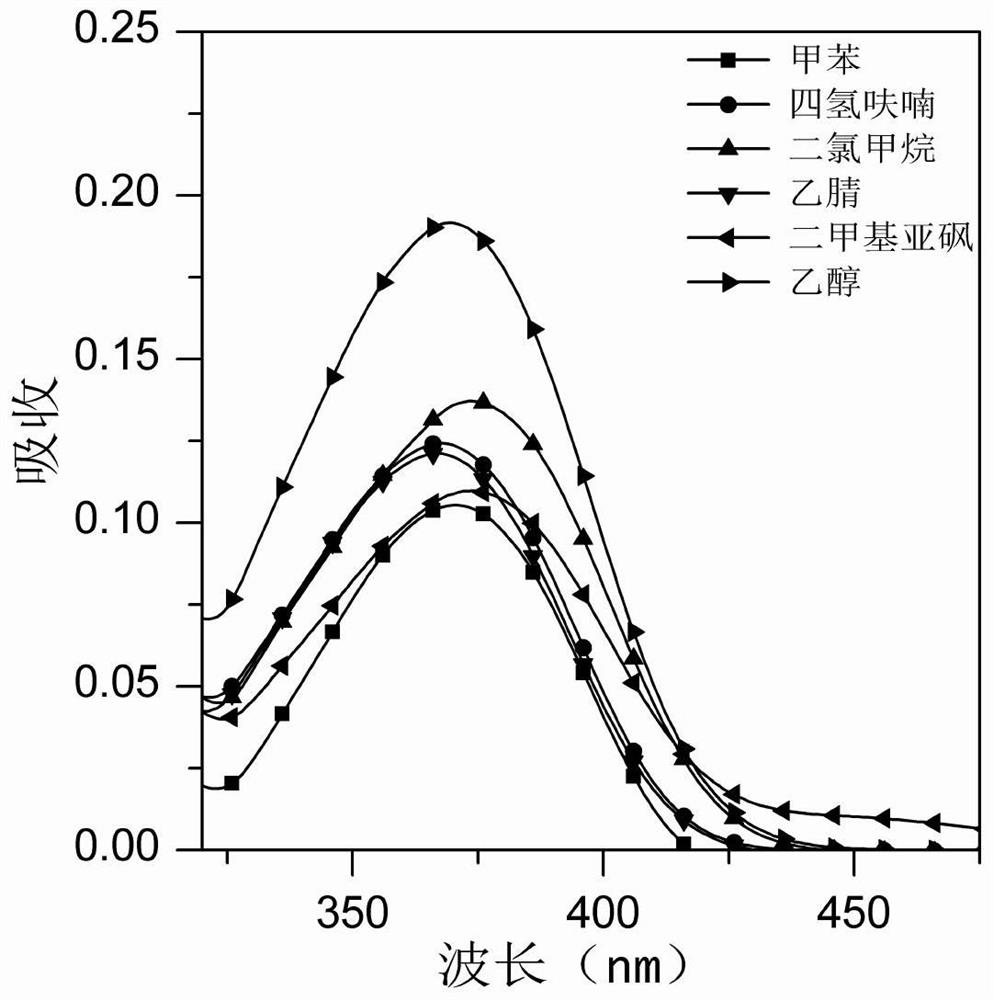
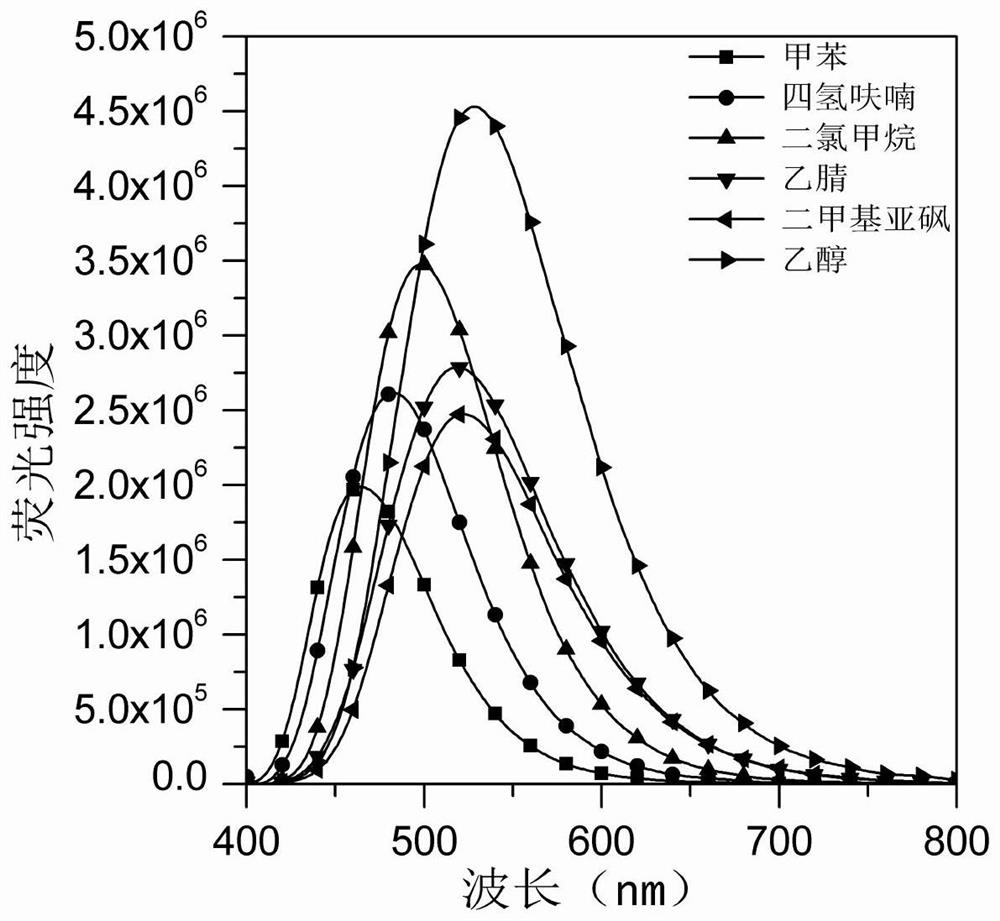
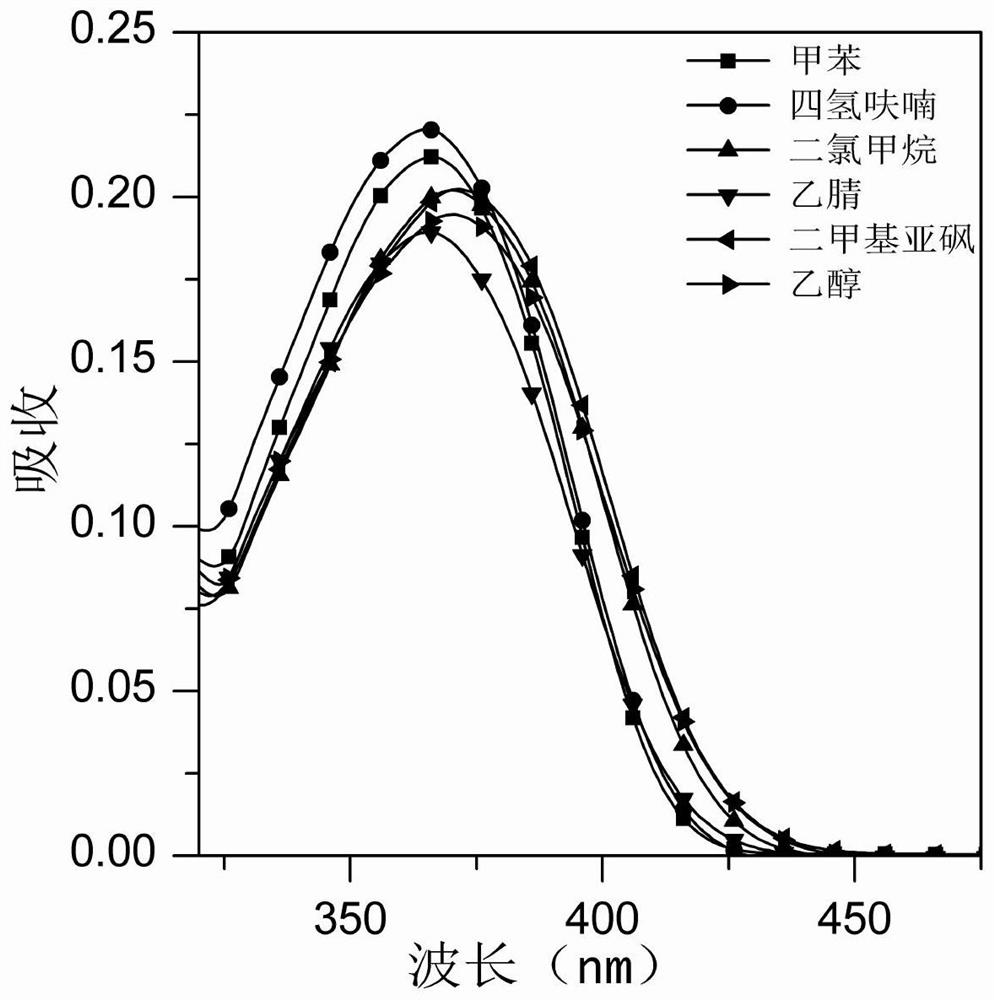
Image
Examples
preparation example Construction
[0052]
[0053] The preparation method of the naphthalimide compound with active end of the present invention comprises the following steps:
[0054] (a), take NH 2 -(CH 2 ) m -OH (m=1-4) or o-, m-, p-aminophenol and 4-bromo-1,8-naphthalene dicarboxylic anhydride dissolved in ethanol and reacted by heating, after the first post-treatment to obtain amides Nitrogen (N) substituted 4-bromo-1,8-naphthalimide;
[0055] (b), 4-bromo-1,8-naphthalimide substituted with nitrogen (N) on the amide obtained in step (a) and 9,9-dimethylfluorene-2-boronic acid are dissolved in a solvent, and The heating reaction is carried out under the catalyst and alkali solution, and the final target compound is obtained through the second post-treatment.
[0056] Wherein, in step (a),
[0057] When R is aminoalcohol NH 2 -(CH 2 ) m When -OH, the specific synthetic route is as follows:
[0058]
[0059] Specifically, 4-bromo-1,8-naphthalic anhydride, NH 2 -(CH 2 ) m The molar volume rati...
Embodiment 1
[0092] Embodiment 1: the synthesis of compound NIR2
[0093]
[0094] In a 250mL three-necked flask equipped with a magnetic stirring bar, 4-bromo-1,8-naphthalic anhydride (compound 1) (2.77g, 10mmol), ethanolamine (670mg, 11mmol) and 150mL ethanol were added successively, and the temperature was raised to 85 ° C, stirred and refluxed for 6h. Naturally cooled to room temperature, poured into a beaker containing 200mL of water and stirred, then left to stand and filtered with suction to obtain a filter cake, washed with saturated NaHCO 3 The solution was washed, dried in vacuo, and recrystallized with 200 mL of absolute ethanol to obtain 2.75 g of light brown powder NIR2 with a yield of 86%. 1 H NMR (400MHz, DMSO-d6) δppm 3.58-3.66(m, 2H), 4.14(t, J=6.53Hz, 2H), 4.86(t, J=6.02Hz, 1H), 8.00(t, J=7.91 Hz, 1H), 8.22 (d, J = 8.03Hz, 1H), 8.32 (d, J = 7.78Hz, 1H), 8.52-8.59 (m, 2H).
Embodiment 2
[0095] Embodiment 2: the synthesis of compound NIRF2
[0096]
[0097] At room temperature, NIR2 (1.6g, 5mmol), 9,9-dimethylfluorene-2-boronic acid (1.19g, 5mmol),
[0098] Pd(PPh 3 ) 4 (57 mg, 0.05 mmol) and 25 mL of DMF were placed in a 50 mL reaction tube. Then join K 2 CO 3 Aqueous solution 5mL (K 2 CO 3 The concentration of the solution was 1.30 mol / L), so that the solid compound was completely dissolved to obtain a brownish-red transparent solution, and the oxygen was removed by bubbling, under nitrogen protection, the temperature was gradually raised to 80° C., and the reaction was carried out for 6 hours. Naturally cooled to room temperature, poured into 100mL of water, and the filter cake obtained by suction filtration was dissolved in dichloromethane (20mL), washed with saturated brine (20mL×2), and the combined organic phase was collected, anhydrous Na 2 SO 4 After drying and spin-drying, the crude product was recrystallized with 200 mL of ethanol to obta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com