Preparation method of near-infrared fluorescent molecule with aggregation-induced emission characteristic
A technology of aggregation-induced luminescence and fluorescent molecules, applied in the field of organic photofunctional materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The synthetic method of above-mentioned compound 1, comprises the following steps:
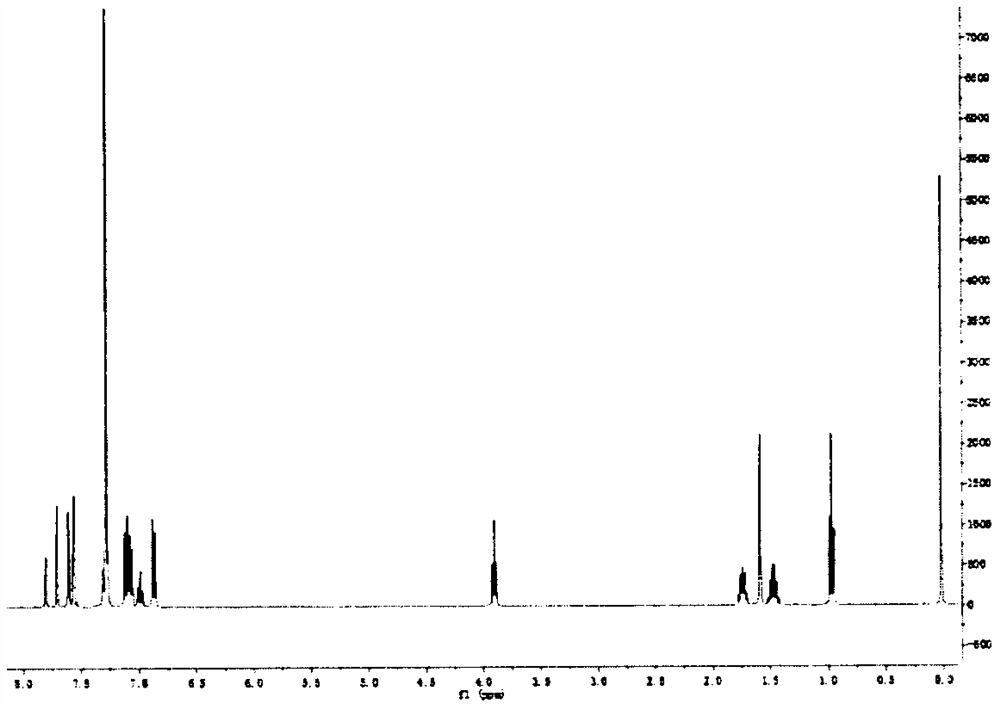
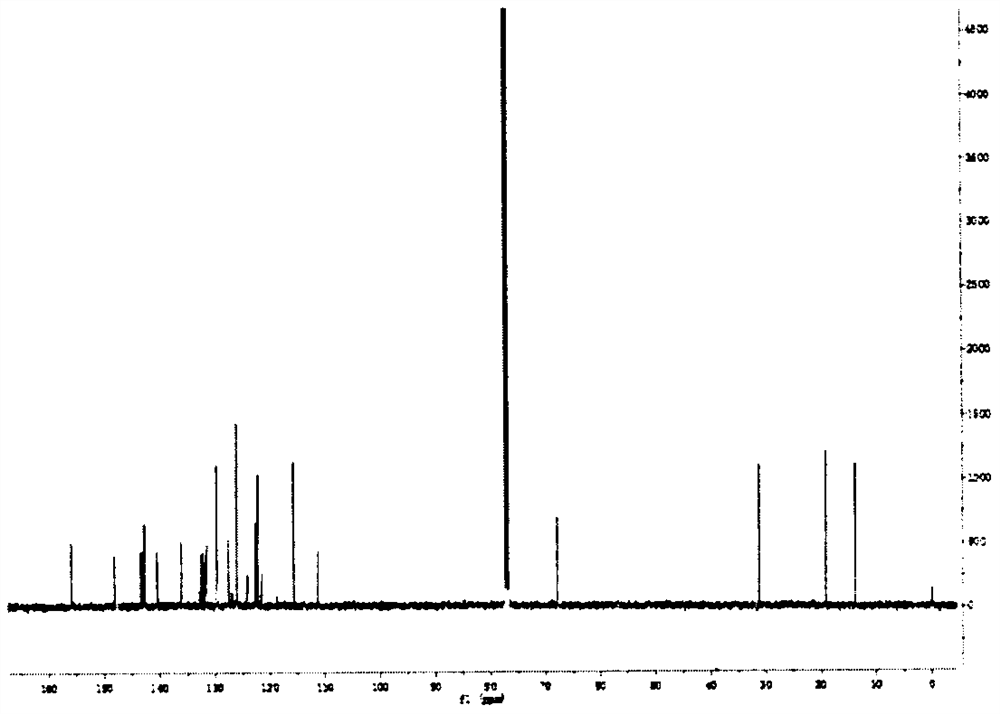
[0021] Compound 1, the molecular formula is C 60 h 46 f 15 N 3 o 2 , the chemical formula of compound 1 is as shown in formula (I):
[0022]
[0023] Its preparation method, the steps are as follows:
[0024] 1) Compound 1 (0.5mmol, 0.381g) and 4-butoxydiphenylamine (2.5mmol, 0.603g), three (dibenzylideneacetone) palladium (0.038mmol, 0.022g), 2-dicyclohexyl Ketone-2',6'-diisopropoxy-1,1'-biphenyl (0.15mmol, 0.070g) and tripotassium phosphate (5.0mmol, 1.061g) were placed in a 25mL two-necked bottle under nitrogen protection , add 2 mL of toluene. 2) Add 0.5ml triethylamine and react at 110°C for 6h;
[0025] 2) React at 110°C for 24 hours. After the system was cooled, dichloromethane (50 mL) was added and extracted three times with water.
[0026] 3) The organic phase was dried overnight with anhydrous magnesium sulfate. The crude product was recrystallized with chlorofor...
Embodiment 2
[0029] The synthetic method comprises the following steps:
[0030] 1) Compound 1 (0.5mmol, 0.381g) and 4-butoxydiphenylamine (2.5mmol, 0.724g), three (dibenzylideneacetone) palladium (0.038mmol, 0.022g), 2-dicyclohexyl Ketone-2',6'-diisopropoxy-1,1'-biphenyl (0.15mmol, 0.070g) and tripotassium phosphate (5.0mmol, 1.061g) were placed in a 25mL two-necked bottle under nitrogen protection , add 2 mL of toluene. 2) Add 0.5ml triethylamine and react at 110°C for 6h;
[0031] 2) React at 110°C for 24 hours. After the system was cooled, dichloromethane (50 mL) was added and extracted three times with water.
[0032] 3) The organic phase was dried overnight with anhydrous magnesium sulfate. The crude product was recrystallized with chloroform / ethanol to obtain the compound (yield 45%)
[0033] The molar ratio of compound 1 to 4-butoxydiphenylamine is 0.5 mmol: 3.0 mmol.
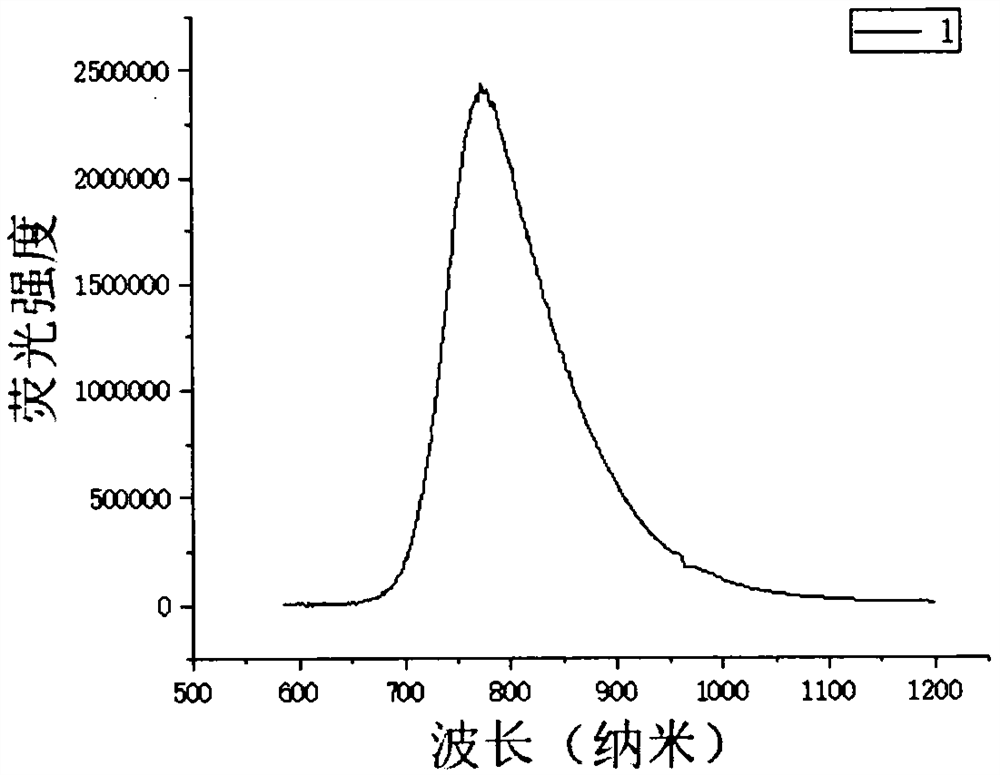
[0034] Characterization 1. Solid-state fluorescence spectrum of fluorescent molecules:
[0035] The fluore...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com