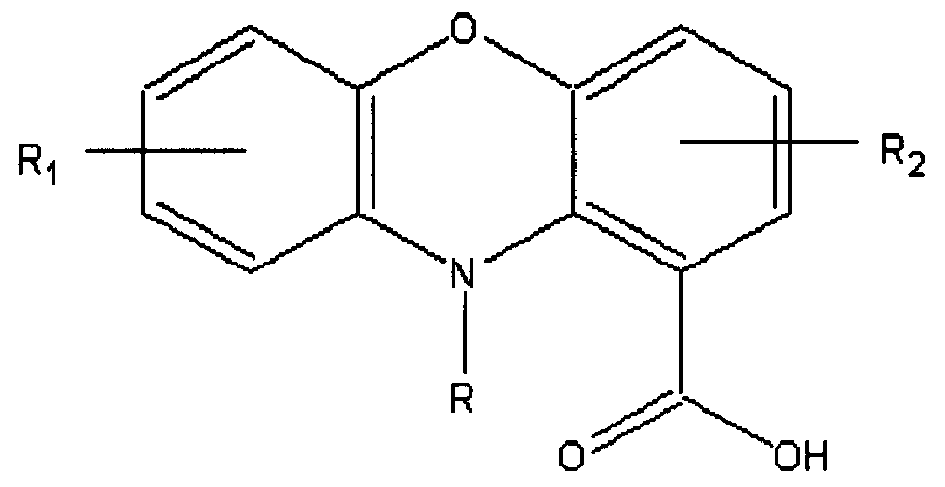
Phenoxazine compounds, its pharmaceutical compositions and medical use
A technology for compounds and prodrugs, applied to phenoxazine compounds and their pharmaceutical compositions and their medical application fields, can solve problems such as aberrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] 3-Nitro-12H-benzo[b]phenoxazine-1-carboxylic acid Preparation Step A: 2-3-Hydroxy-naphthalen-2-yl-amino)-3,5-dinitro-benzoic acid preparation of
[0094] 3-Amino-naphthalene-2-ol (4.65 g, 0.029 mol), 2-chloro-3,5-dinitro-benzoic acid (7.20 g, 0.029 mol), water (30 ml) and 2N sodium acetate (15 mL) was stirred and heated at reflux for 15 minutes. To the resulting thick viscous solution was added 2N sodium hydroxide (15 mL), and the mixture was stirred and heated for 15 minutes. The resulting purple-black sodium salt was washed with cold brine. The filtered precipitated aqueous solution was then acidified with dilute hydrochloric acid. The free acid forms a black precipitate. The precipitate was dissolved in 10% MeOH-H 2 Grinding in the boiling liquid of O, then filtering, with 10% MeOH-H 2 Rinse with cold solution of O and dry overnight in a vacuum oven at room temperature to obtain 9.00 g (0.024 mol, 84%) of the desired product as a brown solid with a melti...
Embodiment 2
[0097] 3-Nitro-8-phenyl-10H-phenoxazine-1-carboxylic acid Preparation Step A: 2-(4-Hydroxy-biphenyl-3-yl-amino)-3,5-dinitro- Preparation of benzoic acid
[0098] The title compound was prepared with 3-amino-biphenyl-4-ol (5.59 g, 0.03 mol), 2-chloro-3,5-dinitro-benzoic acid (7.45 g, 0.03 mol), water (30 mL), 2N sodium acetate (15 mL) and 2N-sodium hydroxide (15 mL) were prepared by the method of step A of Example 1. The desired product was obtained as 9.37 g (0.023 mol, 78%) of a red solid with a melting point of 215-217°C. Elemental Analysis C 19 h 13 N 3 o 7 1.13H 2 O: Calculated: Carbon, 54.90; Hydrogen, 3.70; Nitrogen, 10.11; Found: Carbon, 54.51; Hydrogen, 3.57; Nitrogen, 9.84. Step B: Preparation of 3-nitro-8-phenyl-10H-phenoxazine-1-carboxylic acid
[0099] The title compound was prepared with 2-(4-hydroxy-biphenyl-3-ylamino)-3,5-dinitro-benzoic acid (8.82 g, 0.022 mol), water (50 ml), 10N-hydrogen Sodium oxide (10 ml) was prepared by the method in step B...
Embodiment 3
[0101] Preparation of 3-nitro-10H-phenoxazine-1-carboxylic acid Step A: Preparation of 2-(4-hydroxyphen-3-yl-amino)-3,5-dinitro-benzoic acid
[0102] The title compound was prepared with 3-aminobenzene-4-ol (3.27 g, 0.03 mol), 2-chloro-3,5-dinitro-benzoic acid (7.45 g, 0.03 mol), water (30 ml) , 2N sodium acetate (15 ml) and 2N sodium hydroxide (15 ml) were prepared by the method of step A of Example 1. The desired product was obtained as 9.37 g (0.023 mol, 78%) of a red solid with a melting point of 210-212°C. Elemental Analysis C 13 h 9 N 3 o 7 : Calculated: Carbon, 48.91; Hydrogen, 2.84; Nitrogen, 13.16; Oxygen, 35.08; Found: Carbon, 48.71; Hydrogen, 3.02; Nitrogen, 13.22. Step B: Preparation of 3-nitro-10H-phenoxazine-1-carboxylic acid
[0103] The title compound was prepared with 2-(4-hydroxyphen-3-ylamino)-3,5-dinitro-benzoic acid (5.98 g, 0.022 mol), water (50 ml), 10N sodium hydroxide ( 10 ml) prepared by the method of step B of Example 1 to obtain the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com