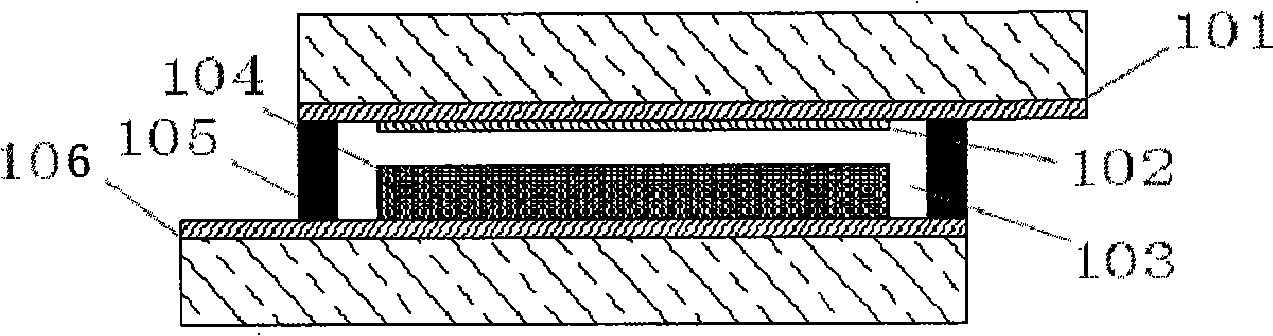
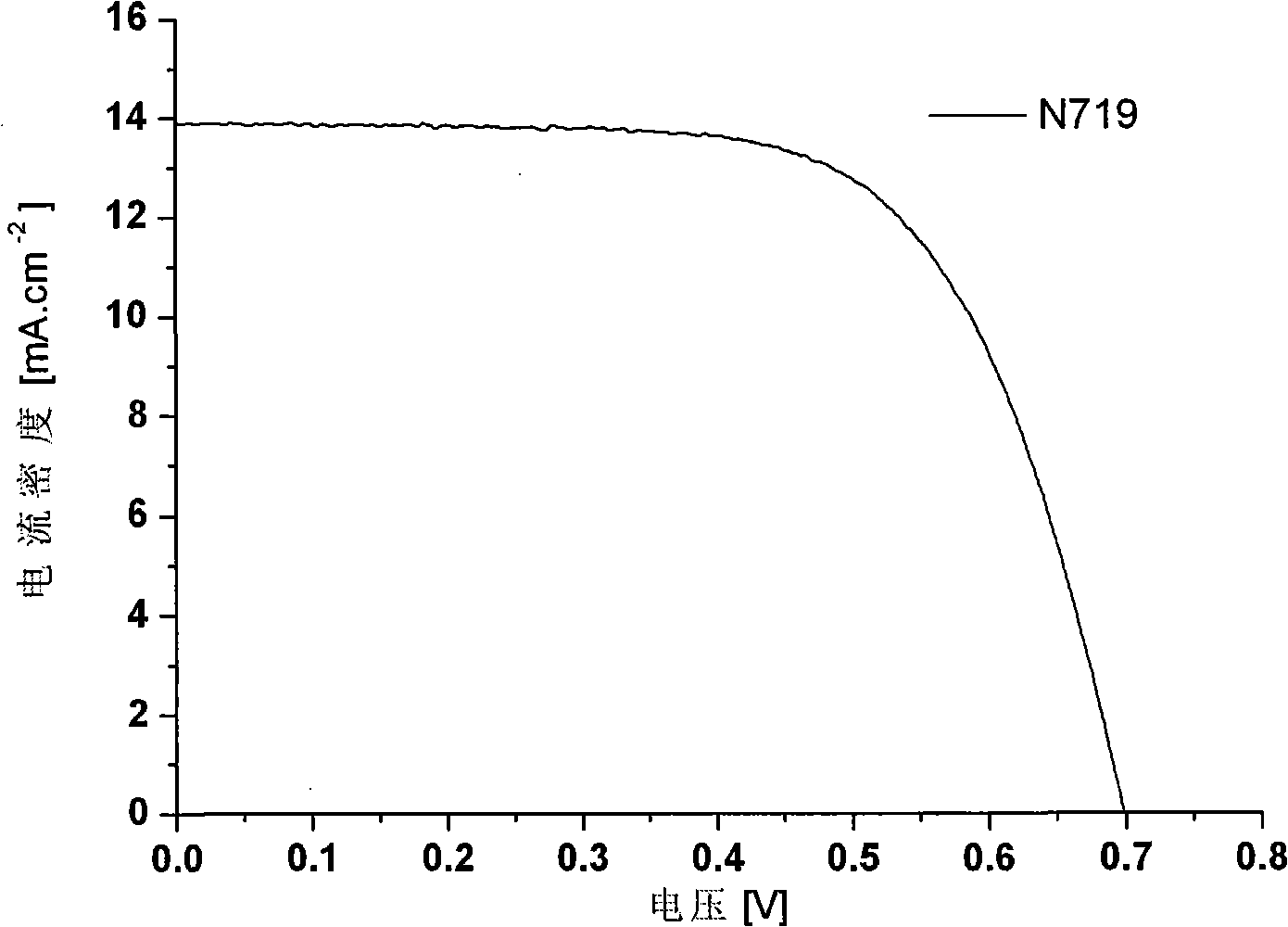
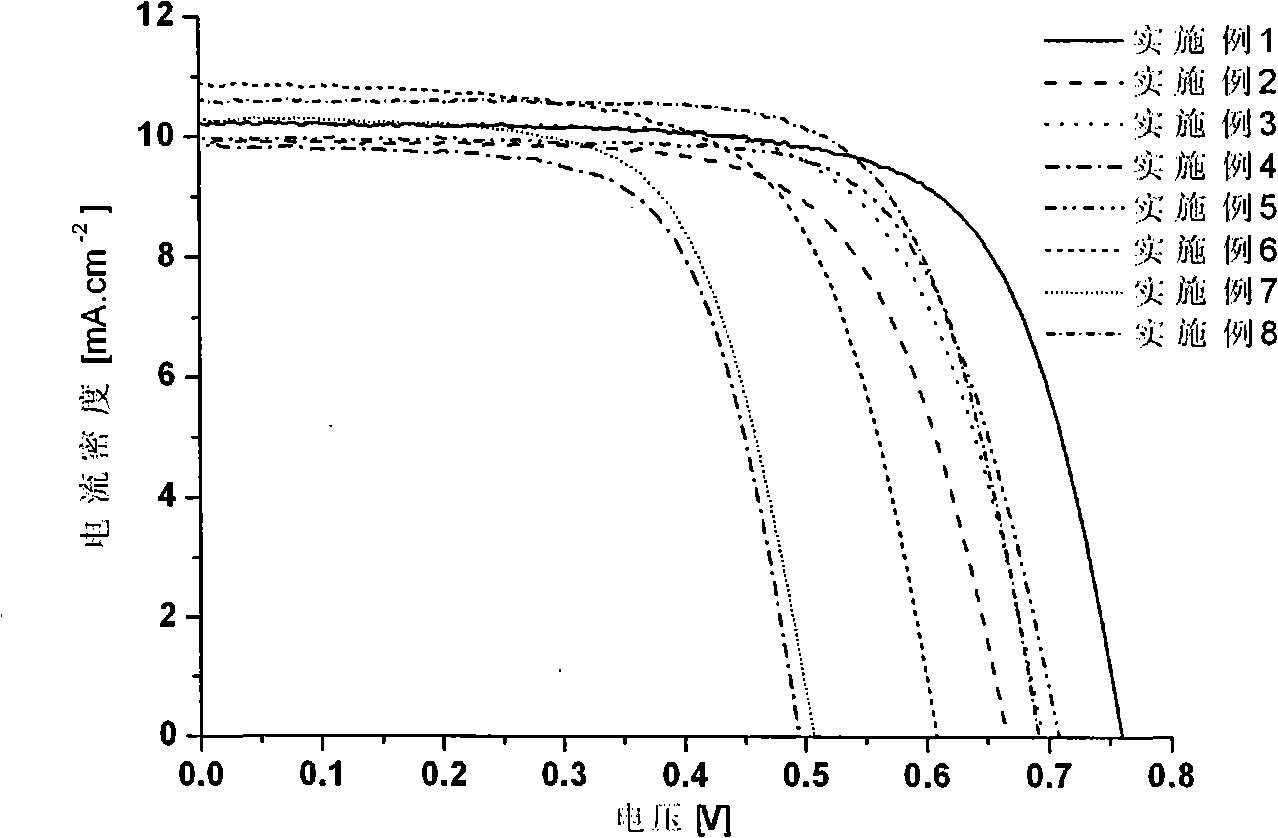
Phenazine oxazines dye and application of the same in dye sensitization of solar battery
A solar cell, dye sensitization technology, applied in the directions of azine dyes, organic dyes, circuits, etc., can solve the problems of high cost and limited application, and achieve the effects of reducing production cost, high molar extinction coefficient, and high photoelectric conversion efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Synthesis of N-dodecyl-3-cyanoacrylic acid group-phenoxazine
[0040] Reaction all carries out in dry environment, specifically comprises the following steps:
[0041] (1) Synthesis of N-dodecyl-3-formyl-phenoxazine
[0042]
[0043] Dissolve 1.05g (3mmol) of N-dodecyl-phenoxazine in 15ml of chloroform, add 548mg of DMF (7.51mmol), stir vigorously at room temperature, and slowly add 2.3g of POCl 3 (15 mmol). Then heat up to reflux state and keep warm for 24 hours. After the reaction, the acetone was evaporated as much as possible by a rotary evaporator. Using dichloromethane as eluent to perform column separation and purification on a silica gel column, 1.02 g (2.69 mmol) of compound N-dodecyl-3-formyl-phenoxazine was obtained, with a yield of 89.7%. NMR l H-NMR (400 MHz, Acetone-d6): δ (ppm): 0.84 (3H, t), 1.25-1.50 (18H, m), 1.70-1.71 (2H, m), 3.65-3.69 (2H, t) , 6.66(1H, d), 6.68-6.89(4H, m), 7.44-7.51(2H, m), 9.52(1H, s). Mass spectrum TOF MS ES ...
Embodiment 2
[0047] Example 2: N-ethyl-3-cyanoacrylic acid-phenoxazine
[0048]
[0049] Synthetic method is basically the same as embodiment 1, nuclear magnetic 1 H-NMR (400 MHz, Acetone-d6): δ (ppm): 1.03 (3H, t), 3.16 (2H, t), 6.72 (1H, d), 6.76-6.92 (4H, m), 7.51-7.57 (2H, m), 8.04 (1H, s). Mass Spectrometry TOF MS ES + : Found m / z 306.1005.Calc.for C 18 h 14 N 2 o 3 : 306.1004.
Embodiment 3
[0050] Example 3: N-Butyl-3-cyanoacrylate-phenoxazine
[0051]
[0052] Synthetic method is basically the same as embodiment 1, nuclear magnetic 1 H-NMR (400 MHz, Acetone-d6): δ (ppm): 0.96 (3H, t), 1.31 (2H, t), 1.48 (2H, t), 3.05 (2H, t), 6.70 (1H, d ), 6.79-6.94 (4H, m), 7.54-7.60 (2H, m), 8.03 (1H, s). Mass Spectrometry TOF MS ES + : Found m / z 334.1320.Calc.for C 20 h 18 N 2 o 3 : 334.1317.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com