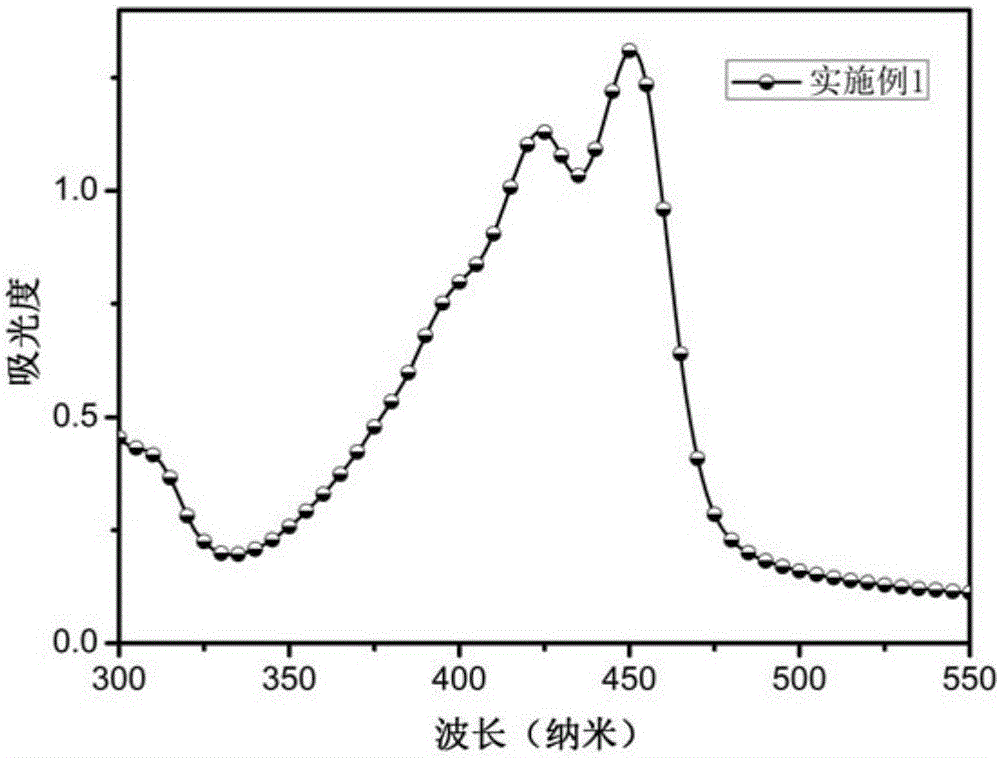
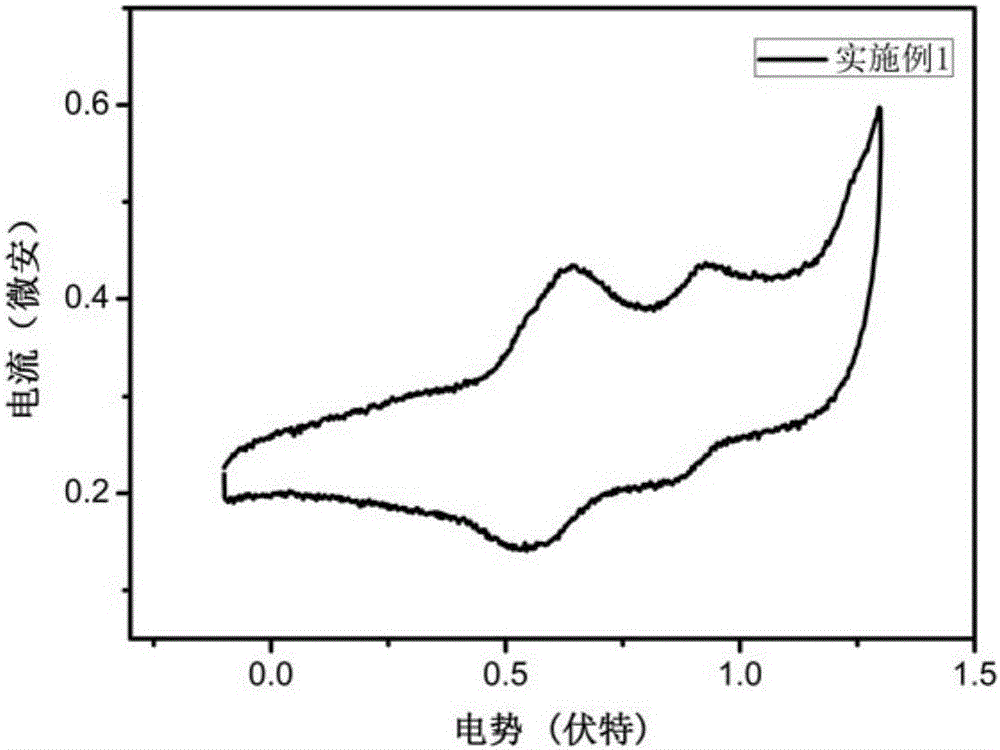
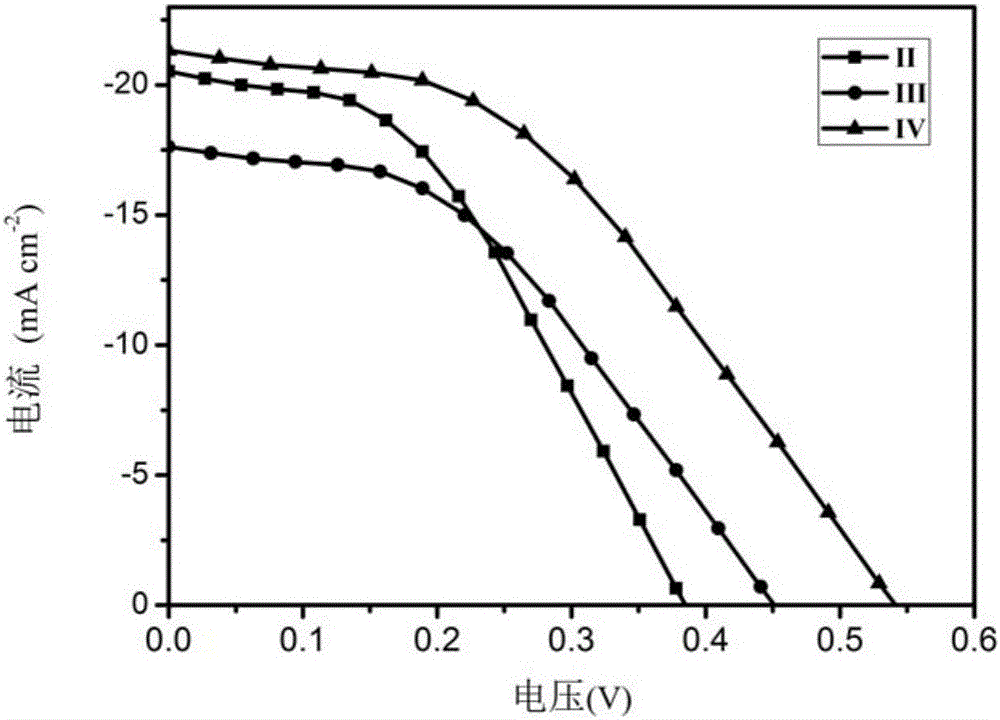
Triphenylamine-benzothiophene organic small-molecule hole transporting material and application thereof
A hole transport material, benzothiophene technology, applied in the field of solar cell materials, can solve problems such as poor stability, and achieve the effect of improving stability, outstanding effect, and broadening the molecular conjugated system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of triphenylamine-benzothiophene organic small molecule hole transport materials with structural units of formula II.
[0047] A kind of triphenylamine-benzothiophene organic small molecule hole transport material with chemical structure II, its synthesis route is as follows:
[0048]
[0049] Synthesis of intermediate (2):
[0050] 2.2 g of compound 1, 1.4 g of zinc powder and 30 mL of water were added to a 100 mL three-necked flask, and then 6 g of NaOH was added, and the mixture was heated to reflux for 1 hour. Then, add 5.0g of bromo-n-hexane and the equivalent of the catalyst tetrabutylammonium bromide (Bu 4 NBr), the reaction was heated to reflux for 6h. The mixture was poured into ice water, extracted twice with 200 mL of THF, the organic phase was dried with anhydrous magnesium sulfate, and after the solvent was removed by rotary evaporation, the initial product was recrystallized with absolute ethanol to obtain white crystals with a yield of 80%.
[0051] NM...
Embodiment 2
[0074] Synthesis of triphenylamine-benzothiophene organic small molecule hole transport materials of chemical structure III.
[0075] A chemical structure formula triphenylamine-benzothiophene organic small molecule hole transport material, and its synthesis route is as follows:
[0076]
[0077] Synthesis of intermediate (6):
[0078] Add 10g 4-iodophenol, 8.9g bromoisooctane, 19g potassium carbonate and 150mL DMF into a 250mL three-necked reaction flask, under nitrogen protection, reflux and stir for 12h at 120°C. Then the mixture was poured into 1M NaOH aqueous solution, extracted with dichloromethane, and the organic liquids were combined, dried over anhydrous magnesium sulfate, and filtered. The solvent was evaporated by rotary evaporation, and the crude product was separated and purified by column chromatography to obtain a colorless liquid with a yield of 53%.
[0079] NMR characterization data of intermediate (6):
[0080] 1 HNMR(CDCl 3 ,500MHz),δ(ppm):7.57(d,J=8.5,2H),6.70(d,...
Embodiment 3
[0102] Synthesis of triphenylamine-benzothiophene organic small molecule hole transport materials of chemical structure IV.
[0103] A kind of triphenylamine-benzothiophene organic small molecule hole transport material of chemical structure IV, its synthesis route is as follows:
[0104]
[0105] Synthesis of intermediate (10):
[0106] Dissolve 5.7g monomer M3 and 2.2g compound 9 in 45mL toluene, then add 2MK 2 CO 3 (12mL) aqueous solution, nitrogen protection, add 0.1mg catalyst Pd(PPh 3 ) 4 , Stir vigorously for 24h at 110℃. The mixture was cooled to room temperature, extracted with dichloromethane, the organic layer was dried over anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure, and separated and purified by column chromatography to obtain Intermediate 10 with a yield of 64%.
[0107] NMR characterization data of intermediate (10):
[0108] 1 HNMR(400MHz, CDCl 3 ,ppm):δ8.75(s2H), 7.80(d,1H), 7.74(d,1H), 7.69-7.65(m,3H), 7.32(d,2H), 7.25(d,4H), 7.01( ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com