Triphenylene compounds, method of manufacturing the same and organic electroluminescent devices employing the same
a technology of triphenylene compounds and organic electroluminescent devices, which is applied in the direction of discharge tube luminescnet screens, natural mineral layered products, etc., can solve the problems of high driving voltage, unknown triphenylene compounds having a silylethyl group, and limited compounds which can be employed, etc., to achieve high excited triplet energy, high heat and light resistance, and high processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
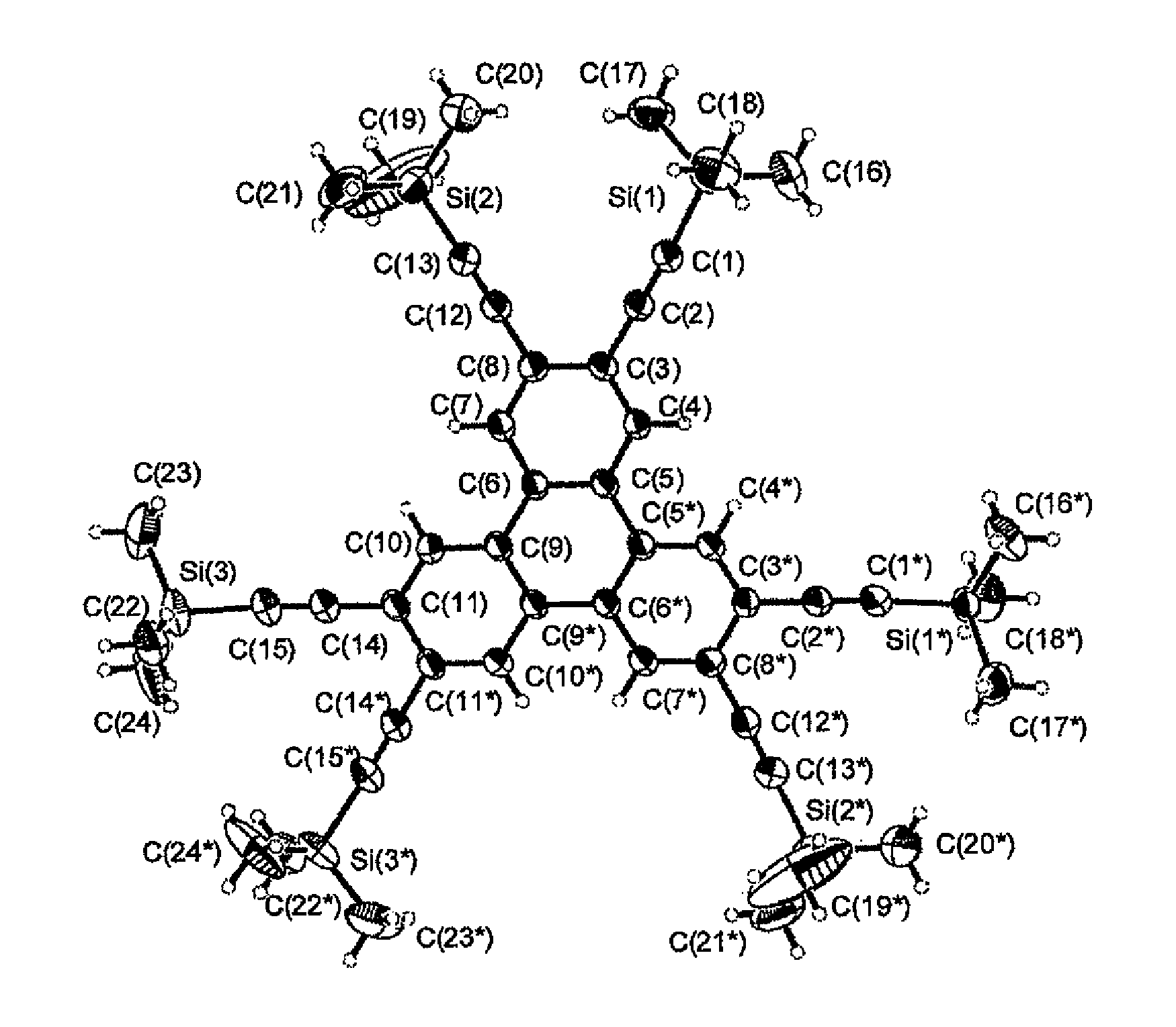
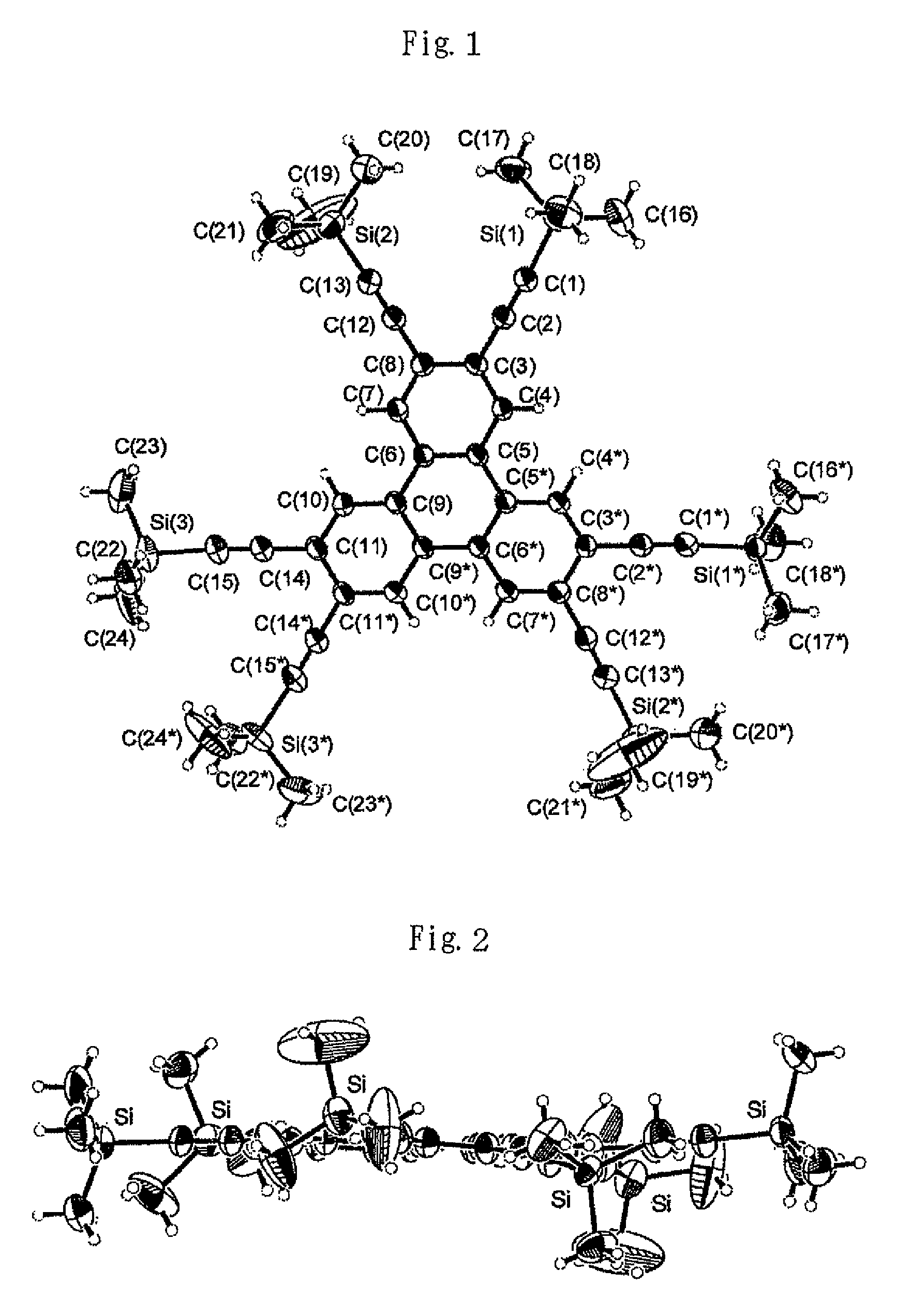
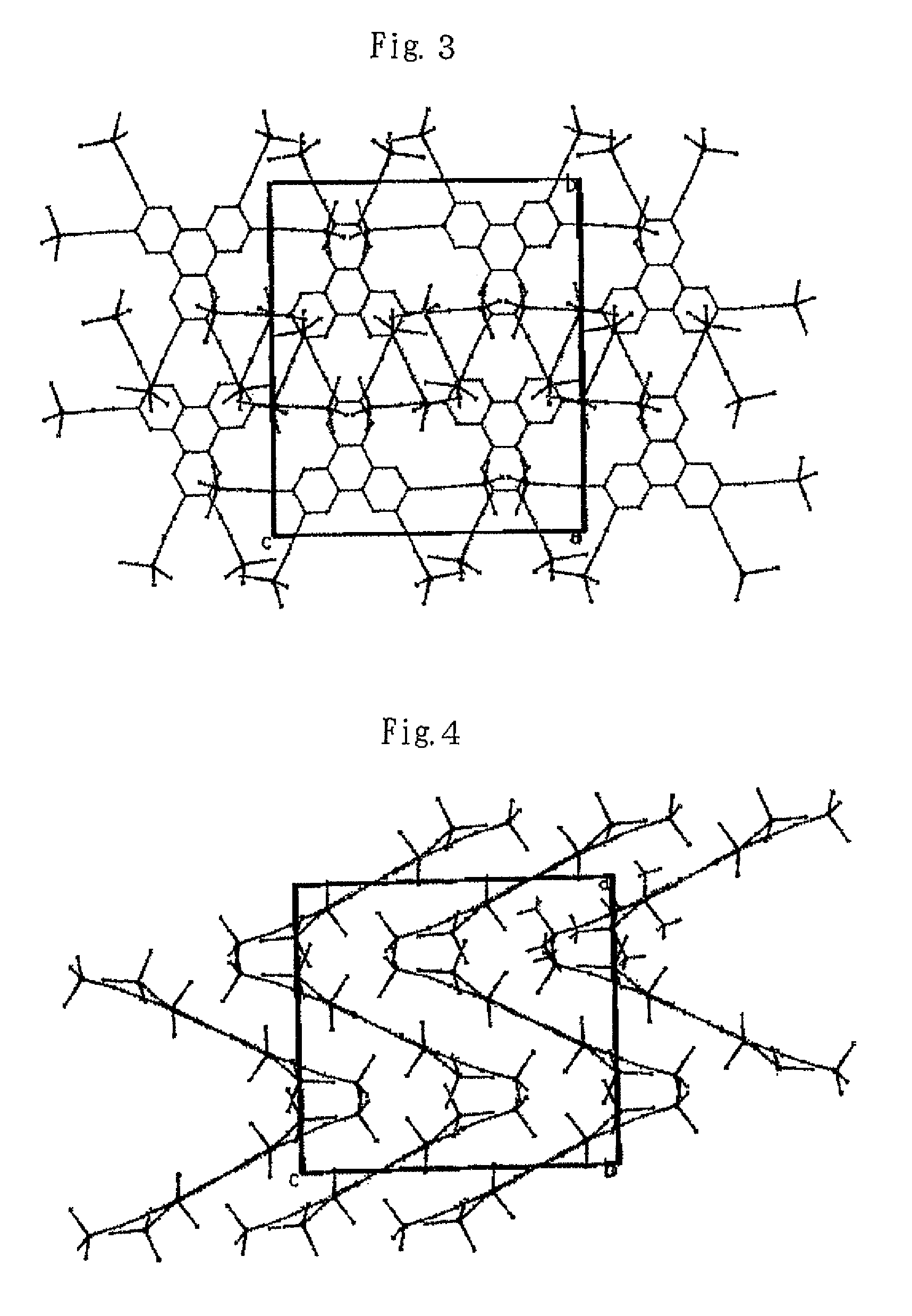
Image
Examples
example 1
1. Synthesis of 2,3,6,7,10,11-Hexakis(trimethylsilylethynyl)triphenylene—General Formula (V)
The synthesis of 2,3,6,7,10,11-hexakis(trimethylsilylethynyl)triphenylene was performed by the method as described below.
1.1. Synthesis of Triphenylene
Triphenylene was synthesized by the reaction shown below:
(Materials and Amounts Employed)
2-Bromofluorobenzene (Tokyo Kasei, 99%): 10.12 g / 5.73×10-z mole
Mg (Wako, flaky, 99.5%): 1.54 g / 6.30×10−2 mole
THF (treated with benzophenoneketyl): 85+15 ml
(Operation and Results)
A 200-ml three-necked flask having a dropping funnel, a Dimroth condenser and a spinner was subjected to flameout treatment and purged with argon. The flask was charged with Mg and it was subjected to two hours of activation at 180° C. The flask was charged with 85 ml of THF, and the dropping funnel with 2-bromofluorobenzene and 15 ml of THF, and their dropping took 20 minutes (the generation of heat started immediately after the dropping was started). The dropping was followed by s...
example 2
Synthesis and Spectral Analysis of 2,3,6,7,10,11-Hexakis(triisopropylsilylethynyl)triphenylene
Triisopropylsilylacetylene was first synthesized by the reaction shown below:
This was reacted with 2,3,6,7,10,11-hexabromotriphenylene to synthesize 2,3,6,7,10,11-hexakis(triisopropylsilylethynyl)triphenylene (general formula (VI)). The reaction was similar to that employed for 2,3,6,7,10,11-hexakis-(trimethylsilylethynyl)triphenylene. The yield was 55%.
The ultraviolet-visible absorption spectra of 2,3,6,7,10,11-hexakis-(triisopropylsilylethynyl)triphenylene were determined in hexane at room temperature. The results are shown in FIG. 10 with data on triphenylene and 2,3,6,7,10,11-hexakis(trimethylsilylethynyl)triphenylene.
A shift to a longer wavelength was observed with 2,3,6,7,10,11-hexakis(triisopropylsilylethynyl)triphenylene, too, as compared with triphenylene.
The fluorescence spectra of 2,3,6,7,10,11-hexakis (triisopropyl-silylethynyl)triphenylene were determined in 3-methylpentane (3-...
example 3
Manufacture of a Phosphorescent Device Including 2,3,6,7,10,11-hexakis-(trimethylsilylethynyl)triphenylene (Compound 1) as a Host Compound and Confirmation of Emission
A transparent supporting substrate was prepared by forming a layer of ITO having a thickness of 150 nm on a glass substrate by vapor deposition. The transparent supporting substrate was fixed to a substrate holder in an evaporator, and the evaporator was furnished with an evaporation boat of molybdenum containing copper phthalocyanine (hereinafter expressed by symbol CuPc), an evaporation boat of molybdenum containing NPD, an evaporation boat of molybdenum containing Ir(ppy)3, an evaporation boat of molybdenum containing Compound 1, an evaporation boat of molybdenum containing BCP, an evaporation boat of molybdenum containing Alq3, an evaporation boat of molybdenum containing lithium fluoride and an evaporation boat of tungsten containing aluminum.
A vacuum tank had its pressure reduced to 1×10−3 Pa, the evaporation boa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| internal quantum efficiency | aaaaa | aaaaa |
| internal quantum efficiency | aaaaa | aaaaa |
| phosphorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com