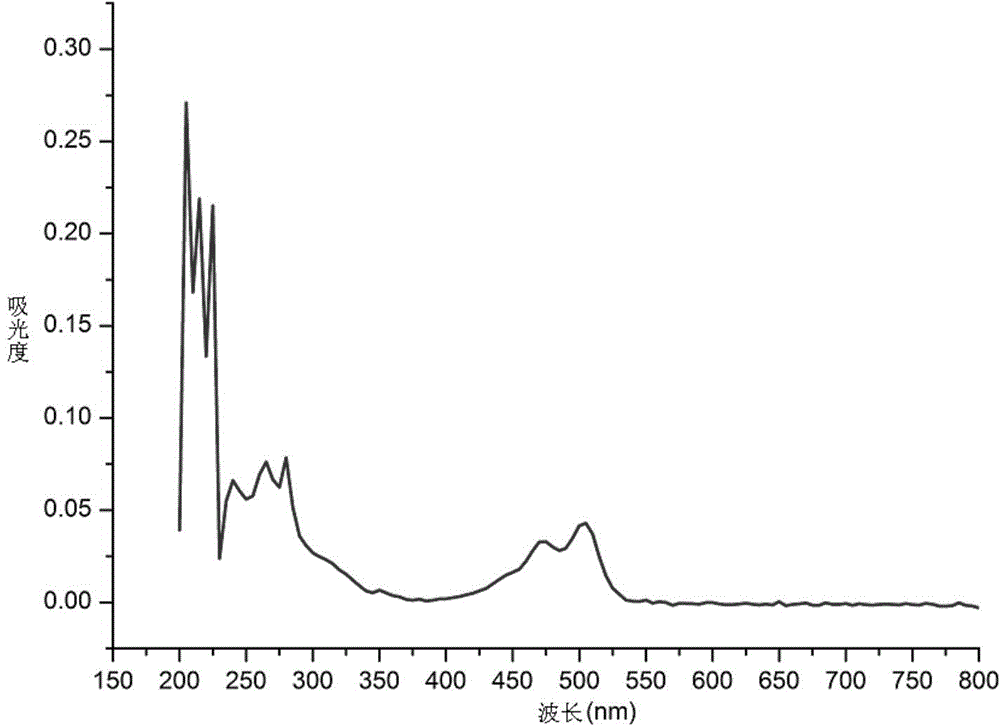
Triphenylene-perylene monoimide diformate binary compound, preparation method and applications thereof
A compound, triphenylene technology, applied in the field of active layers of organic solar cells, can solve the problems of regulation, liquid crystal phase transition temperature and temperature range, etc., and achieve the effect of easy adjustment, easy liquid crystal phase transition temperature and temperature range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Preparation of TP6-C6-ApeDiH
[0067]
[0068] reaction steps
[0069] Compound 1, preparation of o-dihexyloxybenzene
[0070]
[0071] Catechol (20g, 0.1816mol), 1-bromo-n-hexane (89.9g, 0.5448mol), anhydrous potassium carbonate (75.3g, 0.5448mol) and potassium iodide (6.0g) in absolute ethanol (250ml) Reflux for 48 hours. Cool to room temperature and filter with suction. The filter cake was washed with acetone (150ml), filtered with suction, and repeated twice. The filtrate was concentrated, then distilled under reduced pressure, and the fraction at 149-157°C (0.7mmHg) was collected to obtain 48g of compound 1 (colorless oily liquid), with a yield of 96%. 1 HNMR (300MHz, CDCl 3 )δ:6.89(s,4H),3.99(t,4H,J=6.9Hz),1.83-1.76(m,4H,J=6.9Hz),1.49-1.31(m,12H),0.9(t,6H , J=6.9Hz).
[0072] Compound 2, Preparation of 2-hexyloxyphenol
[0073]
[0074] Catechol (50g, 0.45mol), 1-bromo-n-hexane (75g, 0.45mol), anhydrous potassium carbonate (100g, 0.725mol) and pot...
Embodiment 2
[0100] Preparation of TP6-C10-ApeDiH
[0101]
[0102] The preparation of compound 2-(10'-aminodecyloxy)-3,6,7,10,11-penta(hexyloxy)triphenylene refers to the preparation of compound 7 in Experimental Example 1. The only difference is that Br(CH 2 ) 6 Br uses Br(CH 2 ) 10 Br alternative.
[0103] Compound 2-(10’-aminodecyloxy)-3,6,7,10,11-penta(hexyloxy)triphenylene 1 HNMR (300MHz, CDCl 3 )δ:7.84(s,6H),4.26-4.17(m,12H),2.67(t,2H,J=6.9Hz),2.00-1.90(m,12H),1.66-1.32(m,44H),0.94 (t, 15H, J = 6.9 Hz).
[0104] For the preparation of the compound perylenetetracarboxylic monoanhydride diester, see the preparation of compound 9 in Experimental Example 1.
[0105]
[0106] N 2 Under protected conditions, 2-(10'-aminodecyloxy)-3,6,7,10,11-penta(hexyloxy)triphenylene (0.39g, 0.435mmol), perylene monoanhydride diester (0.17g, 0.29mmol) and imidazole (10.5g) were heated to 110°C for 1 hour. Then washed with water, the crude product was purified by silica gel column (eluen...
Embodiment 3
[0113] Preparation of compound TP6-C12-ApeDiH
[0114]
[0115] For the preparation method, refer to the preparation method in Example 1. The only difference is that Br(CH 2 ) 6 Br uses Br(CH 2 ) 12 Br alternative.
[0116] of the compound 1 HNMR (500MHz, CDCl 3 )δ: 8.38(d, 2H, J=10Hz), 8.19(q, 4H), 7.96(d, 2H), 7.63(t, 4H), 7.54(s, 2H), 4.27(t, 4H), 4.14 -4.05 (m, 14H), 1.89-1.81 (m, 12H), 1.77-1.66 (m, 8H), 1.33-1.29 (m, 56H), 0.88-0.83 (m, 21H).
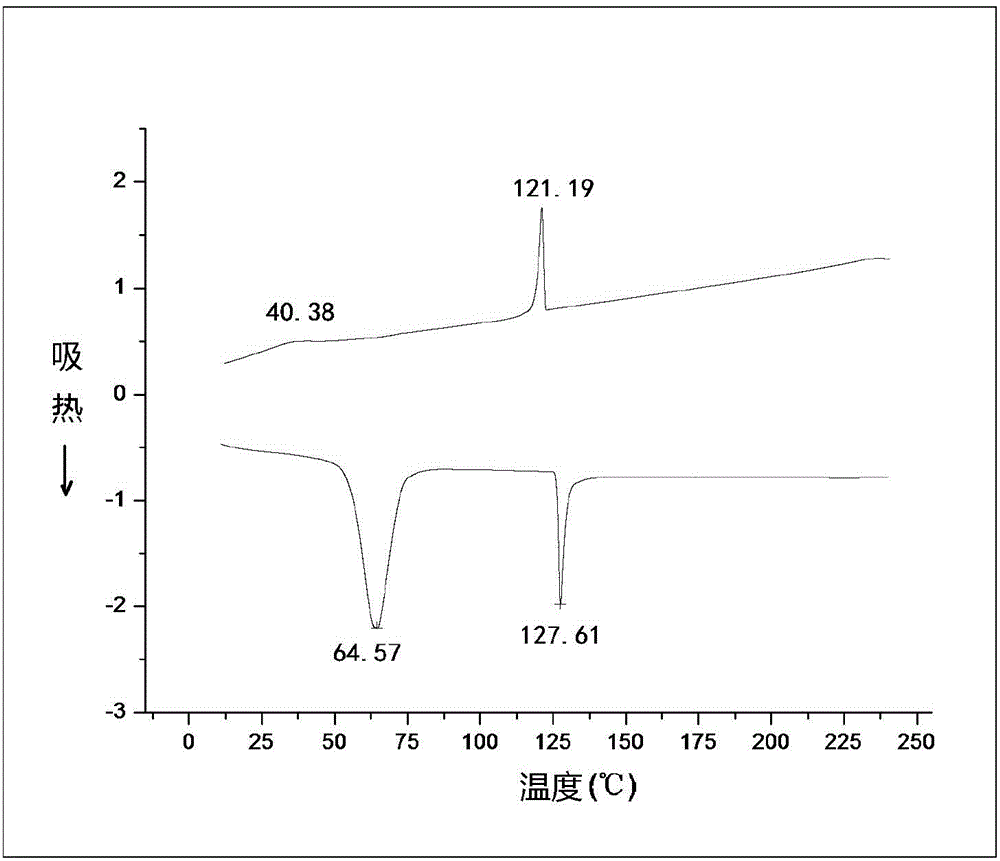
[0117] Figure 4 is the DSC trace of compound TP6-C12-ApeDiH. During the heating process of compound TP6-C12-ApeDiH, 73.1℃ is the melting point, at which point the crystal starts to change into liquid crystal; 113.6℃ is the clearing point, at which time the liquid crystal becomes isotropic liquid; Change from isotropic liquid to liquid crystal, continue to cool down, solidify at 62.8°C, and change from liquid crystal to crystal. The used DSC thermal analyzer (model Q600) is produced by ThermalAnalysis Company of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com