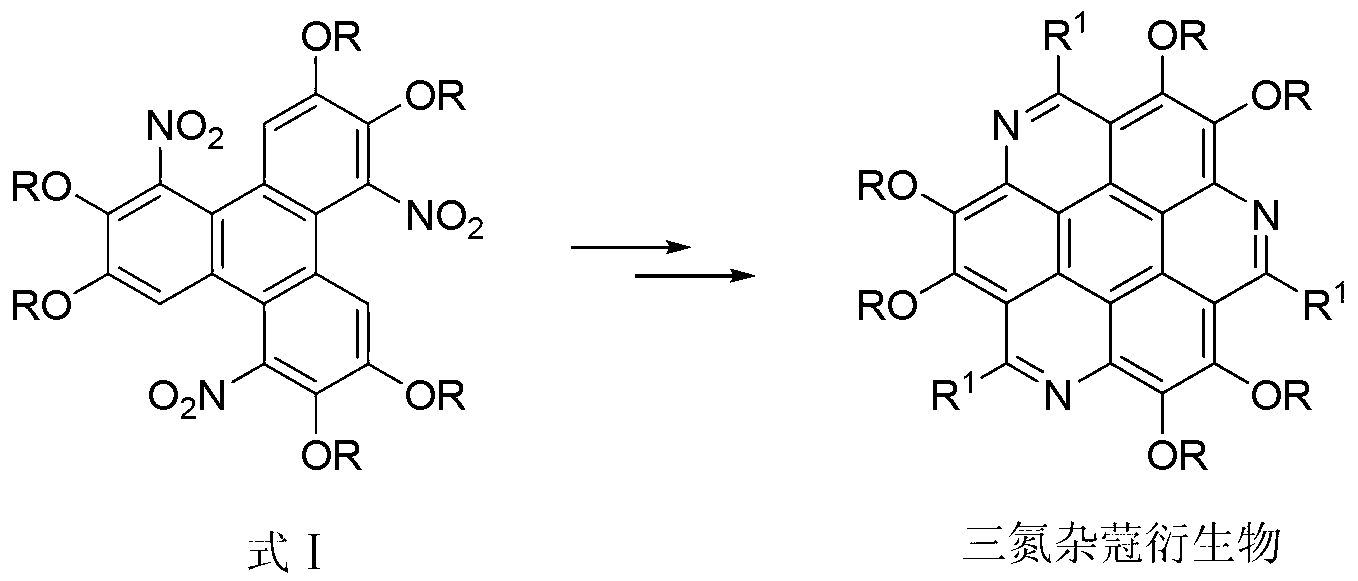
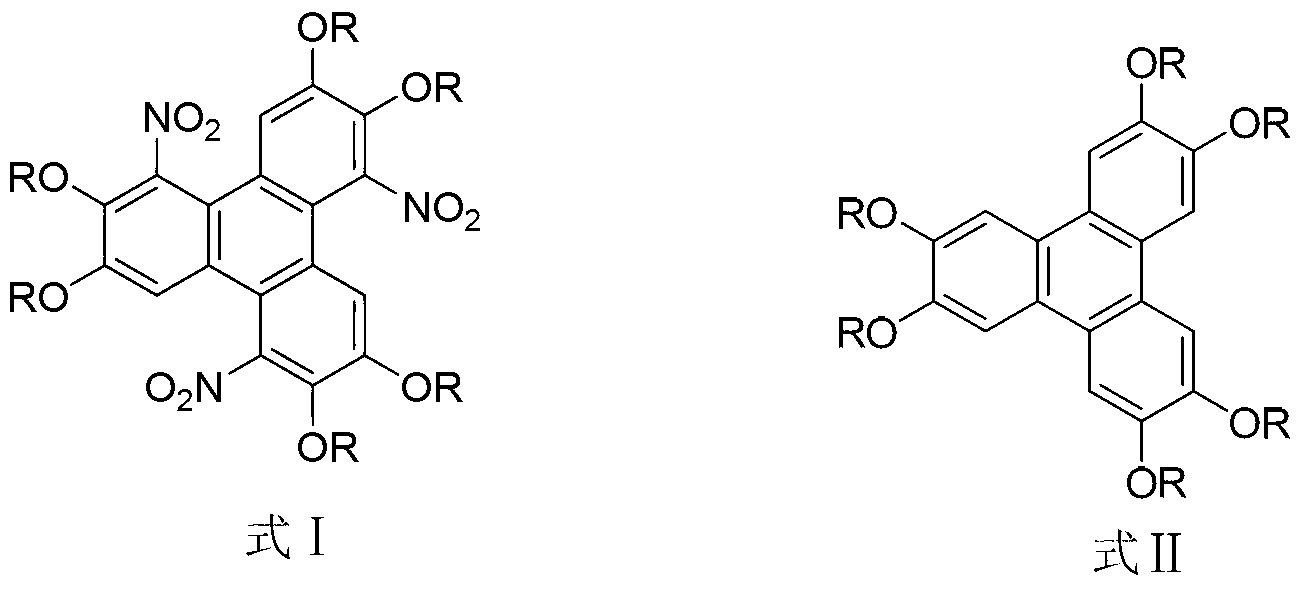
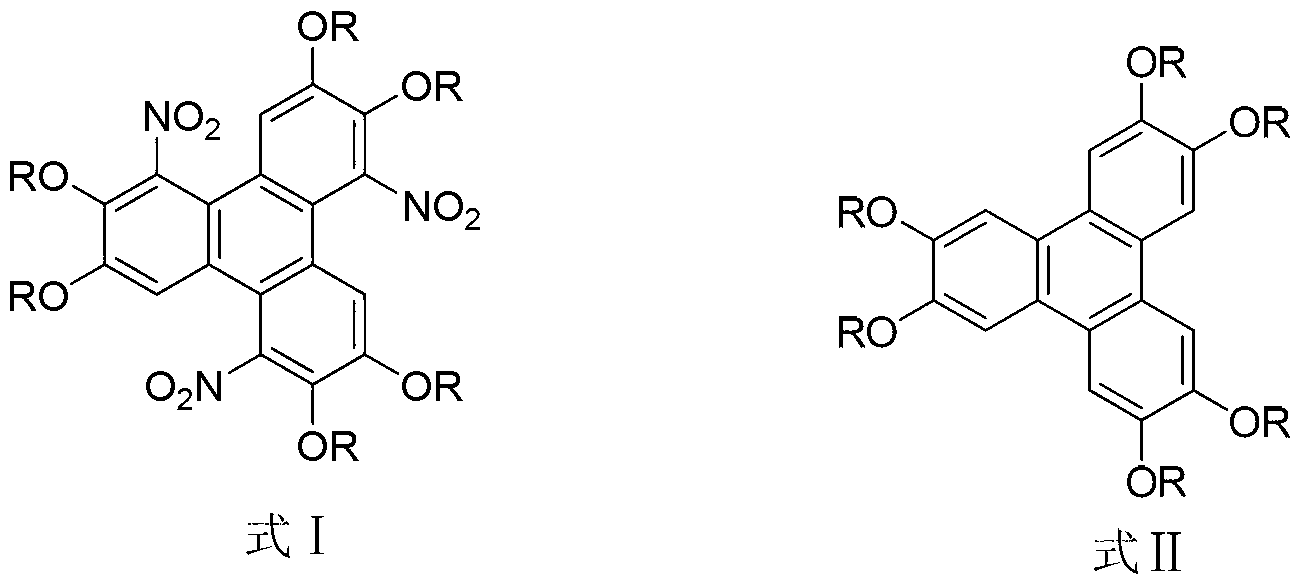
Method for synthesising 1,5,9-trinitro-2,3,6,7,10,11-hexa-alkoxy triphenylene
A technology of hexaalkoxytriphenylene and a synthesis method, which is applied in the preparation of nitro compounds, organic chemistry and other directions, can solve the problems of many by-products, environmental pollution, and product yield of only 28%, and achieves simple operation and reaction The effect of mild conditions and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11
[0016] Synthesis of embodiment 11,5,9-trinitro-2,3,6,7,10,11-hexamethoxytriphenylene
[0017] Dissolve 0.51g (1.25mmol) of 2,3,6,7,10,11-hexamethoxytriphenylene in a flask filled with 10mL of chloroform, add 1.33g (7.5mmol) of benzenesulfonyl chloride, 1.27g of ( 7.5mmol) silver nitrate, the molar ratio of 2,3,6,7,10,11-hexamethoxytriphenylene to benzenesulfonyl chloride and silver nitrate is 1:6:6, stirred and reacted at 60°C for 48 hours, Then the reaction solution was cooled to room temperature, 10 mL of distilled water was added, extracted with chloroform (50 mL×3), and the organic phase was washed with saturated Na 2 CO 3 solution, washed with distilled water and anhydrous Na 2 SO 4 After drying, the product was separated and purified by silica gel column chromatography (trichloromethane eluent) to obtain a pale yellow solid 1,5,9-trinitro-2,3,6,7,10,11-hexamethoxy Triphenylene, its yield is 76%. The spectral data of the product are as follows:
[0018] IR (KBr) ν (...
Embodiment 21
[0022] Synthesis of Example 21,5,9-trinitro-2,3,6,7,10,11-hexamethoxytriphenylene
[0023] Dissolve 0.51g (1.25mmol) of 2,3,6,7,10,11-hexamethoxytriphenylene in a flask containing 10mL of chloroform, and add 1.66g (7.5mmol) of 4-nitrobenzenesulfonyl chloride , 1.27g (7.5mmol) silver nitrate, 2,3,6,7,10,11-hexamethoxytriphenylene and 4-nitrobenzenesulfonyl chloride, the molar ratio of nitrate in silver nitrate is 1:6:6 , stirred and reacted at 60°C for 24 hours, and the other steps were the same as in Example 1 to obtain light yellow solid 1,5,9-trinitro-2,3,6,7,10,11-hexamethoxytriphenylene, which was recovered The rate is 45%.
Embodiment 31
[0024] Synthesis of Example 31,5,9-trinitro-2,3,6,7,10,11-hexamethoxytriphenylene
[0025] Dissolve 0.51g (1.25mmol) of 2,3,6,7,10,11-hexamethoxytriphenylene in a flask filled with 10mL of o-dichlorobenzene, add 1.43g (7.5mmol) of 4-methylbenzenesulfonate Acyl chloride, 0.25g (1.50mmol) silver nitrate, 0.97g (3.00mmol) mercury nitrate, 2,3,6,7,10,11-hexamethoxytriphenylene and 4-methylbenzenesulfonyl chloride, mercury nitrate and nitric acid The molar ratio of nitrate in silver is 1:6:6, and the reaction is stirred at 60°C for 56 hours. The other steps are the same as in Example 1 to obtain a light yellow solid 1,5,9-trinitro-2,3,6,7 , 10,11-hexamethoxytriphenylene, the yield was 48%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com