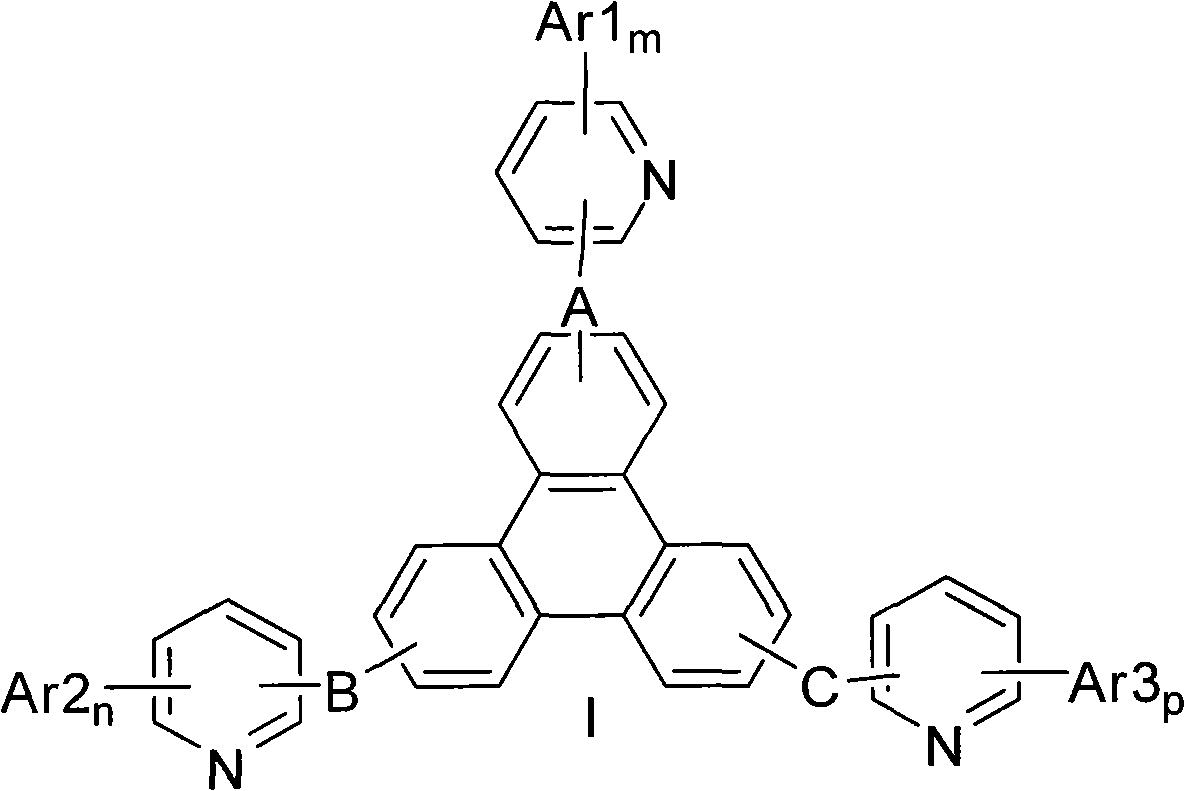
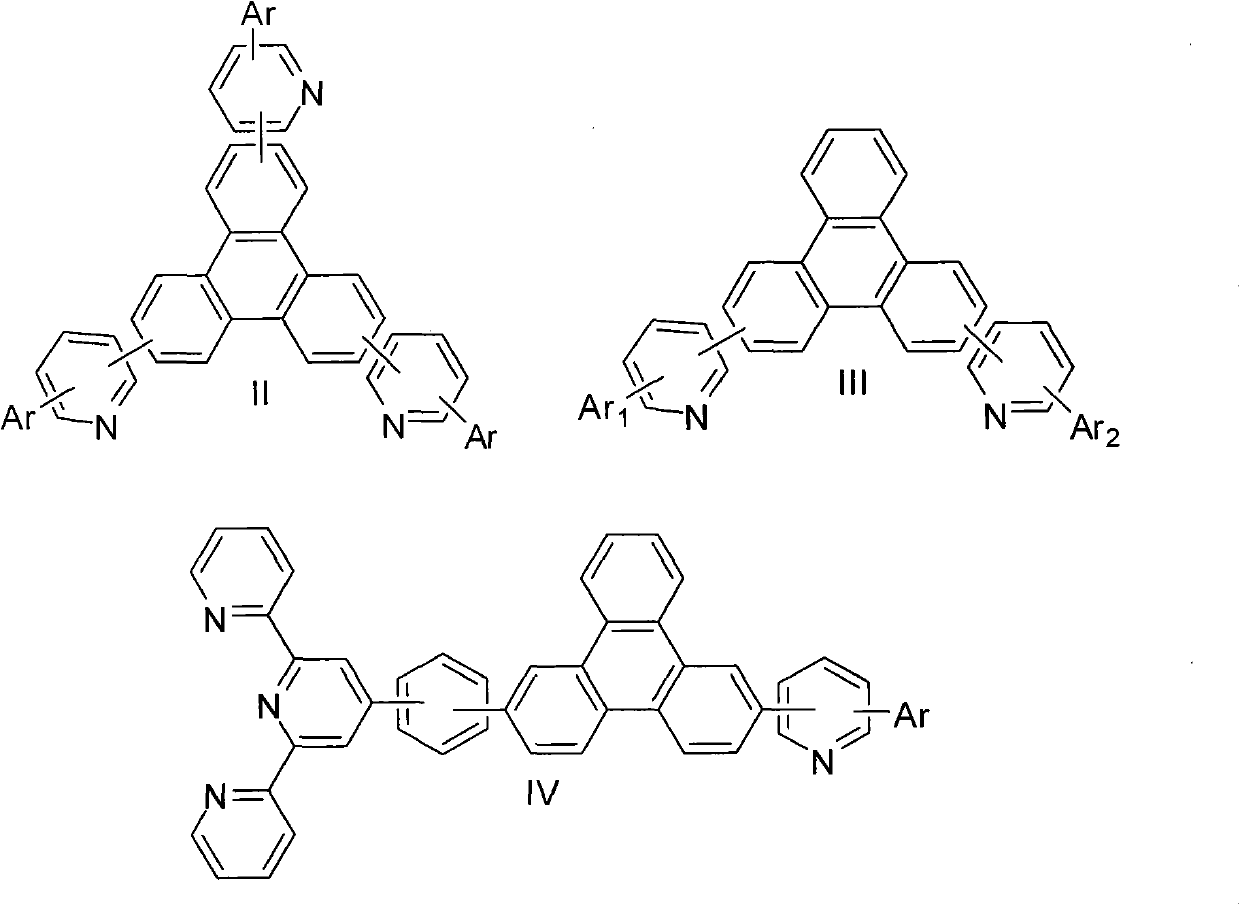
Triphenylene compound containing pyridine group and application thereof
A technology of organic compounds and compounds, applied in organic chemistry, electrical components, circuits, etc., can solve problems such as suppression of endothermic energy transfer process, short life of blue phosphorescent devices, and reduced device efficiency, so as to achieve high electron transport performance and improve Good electron transport performance and film-forming properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 compound 1-1
[0039] (1) Preparation of aryl-substituted pyridine boronic acid:
[0040]
[0041] 23.5g (0.10mmol) 2,6-dibromopyridine, 12.0g phenylboronic acid and 0.50g Pd (PPh 3 ) 4 Dissolve in 300.0mL toluene, dissolve 22.0g potassium carbonate in 100.0mL water and add to the above reaction solution, the reaction solution immediately turns yellow at 50°C. As the reaction progressed, the color of the reaction solution gradually became lighter. After 1.5 h, 2.50 g of phenylboronic acid was added, and the reaction progress was monitored by TLC. After about 0.5h, the reaction was completed, and the organic layer was washed three times with anhydrous Na 2 SO 4 Carry out column chromatography after drying, eluent is sherwood oil: dichloro=20: 1 (V 1 / V 2 ) to obtain 15.1 g of off-white solid. MS (m / e): 234, melting point 47-49°, yield 64.3%. Dissolve the obtained product in 200.0 mL of dry tetrahydrofuran, then add 16.0 g of tri...
Embodiment 2
[0046] The synthesis of embodiment 2 compound 1-2
[0047] Using 2-chloro-4-iodopyridine and phenylboronic acid as raw materials, compound 1-2 was obtained through the same reaction as in Example 1. MS (m / e): 687, elemental analysis (C 51 h 33 N 3 ): theoretical value C: 89.06%, H: 4.84%, N: 6.11%; measured value C: 89.10%, H: 4.71%, N: 6.19%. Yield 62.4%.
Embodiment 3
[0048] The synthesis of embodiment 3 compound 1-3
[0049] Using 3,5-dibromopyridine and phenylboronic acid as raw materials, compound 1-3 was obtained through the same reaction as in Example 1. MS (m / e): 687, elemental analysis (C 51 h 33 N 3): theoretical value C: 89.06%, H: 4.84%, N: 6.11%; measured value C: 89.15%, H: 4.81%, N: 6.04%. Yield 58.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com