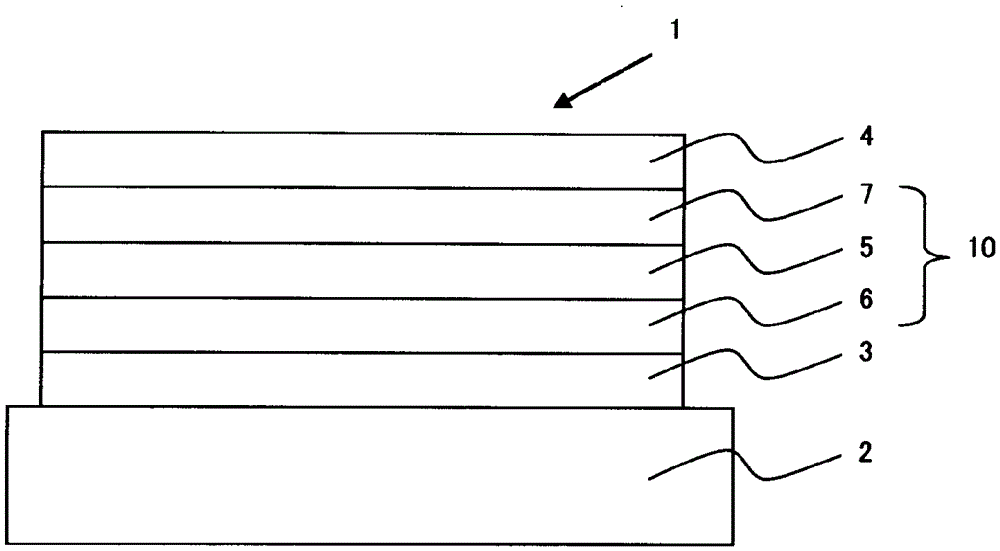
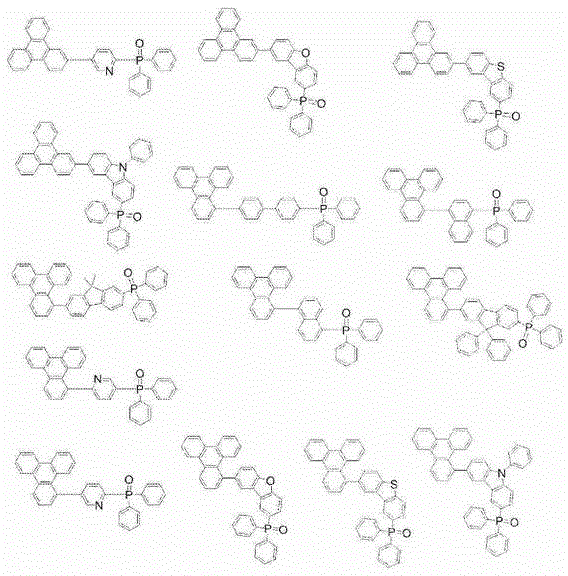
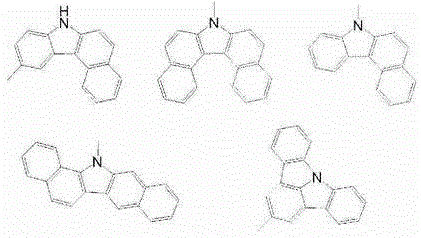
Compound, material for organic electroluminescent elements, organic electroluminescent element and electronic device
A compound and atomic number technology, applied in the field of compounds, to achieve high performance, good driving voltage and external quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0272] [chemical 40]
[0273]
[0274] Under an argon atmosphere, benzo[g]?-10-boronic acid (2.90g, 9.00mmol), (4-bromophenyl)phosphine oxide (3.21g, 9.00mmol), tris(dibenzylideneacetone) di Add 1,4-dioxane (100 mL) to palladium (0) (124 mg, 0.135 mmol), triphenylphosphine (283 mg, 1.08 mmol), and tripotassium phosphate (11.5 g, 54.0 mmol), and stir at 100 ° C for 8 Hour.
[0275] After the reaction, the mixture was diluted with water and extracted with chloroform. Next, the mixture was washed with saturated brine, dried over magnesium sulfate, and concentrated. Then, the mixture was purified by silica gel column chromatography, and then recrystallized from ethyl acetate to obtain a compound (3.24 g, 5.85 mmol, yield 65%). The result of mass analysis of this compound was m / e=554, and it was identified as the above-mentioned compound (1) (Exactmass: 554.18).
Embodiment 2
[0277] [chem 41]
[0278]
[0279] (2-1) Synthesis of intermediate (A2)
[0280] Under an argon atmosphere, raw material compound (A1) (2.00 g, 5.60 mmol) and tetrahydrofuran (50 mL) were mixed, and cooled to -78°C. Then, n-butyllithium (1.60 M hexane solution, 3.68 mL, 5.88 mmol) was added, and the temperature was raised to 0° C. over 2 hours. Next, it cooled to -78 degreeC again, trimethoxyborane (1.45g, 14.0mmol) was added, it stirred at -78 degreeC for 10 minutes, and it heated up to room temperature over 6 hours.
[0281] After the reaction, aqueous hydrochloric acid (1M, 15 mL) was added, stirred at room temperature for 1 hour, and extracted with ethyl acetate. The solution was dried over magnesium sulfate, concentrated, suspended and washed in hexane, and recovered by filtration to obtain intermediate (A2) (902 mg, 2.80 mmol, yield 50%).
[0282] (2-2) Synthesis of compound (2)
[0283] Under argon atmosphere, intermediate (A2) (850mg, 2.64mmol), (4-bromophenyl)p...
Embodiment 3
[0286] [chem 42]
[0287]
[0288] (3-1) Synthesis of intermediate (B1)
[0289] The synthesis of the intermediate (B1) was carried out as described above with reference to the synthesis example described in "J. Org. Chem., 1991, 56, p. 1210-1217".
[0290] (3-2) Synthesis of intermediate (B2)
[0291] Add carbon tetrachloride to intermediate (B1) (2.40g, 8.66mmol), N-bromosuccinimide (1.54g, 8.66mmol), iron(III) chloride hexahydrate (70mg, 0.260mmol) (600mL), stirred under reflux for 8 hours.
[0292] After completion of the reaction, the solvent was distilled off under reduced pressure, and the mixture was purified by silica gel column chromatography to obtain an intermediate (B2) (2.13 g, 5.98 mmol, yield 69%).
[0293] (3-3) Synthesis of intermediate (B3)
[0294] The intermediate (B2) (1.80 g, 5.04 mmol) and tetrahydrofuran (40 mL) were mixed under an argon atmosphere, and cooled to -78°C. Then, n-BuLi (1.60 M hexane solution, 3.31 mL, 5.29 mmol) was added, and th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Film thickness | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com