Preparation method of coronene
A coupling reaction and equivalent technology, which is applied in the preparation of organic compounds, chemical instruments and methods, and the production of hydrocarbons from oxygen-containing organic compounds, etc., can solve the problems of cumbersome operations, harsh conditions, and expensive raw materials, and achieve simple processes and efficient reactions. The effect of mild conditions and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] According to the preparation method of coronene of the present invention, the method comprises:
[0032] Substitution reaction: use 1 equivalent of the compound shown in formula I and 4.2 to 5.6 equivalents of the compound shown in formula II in the presence of catalysts and auxiliary agents to carry out a coupling reaction in a solvent to convert it into a compound shown in formula III;
[0033] Ring-closing reaction: the compound shown in formula III undergoes ring-closing reaction in the presence of acid, and is converted into coronene shown in formula IV;
[0034]
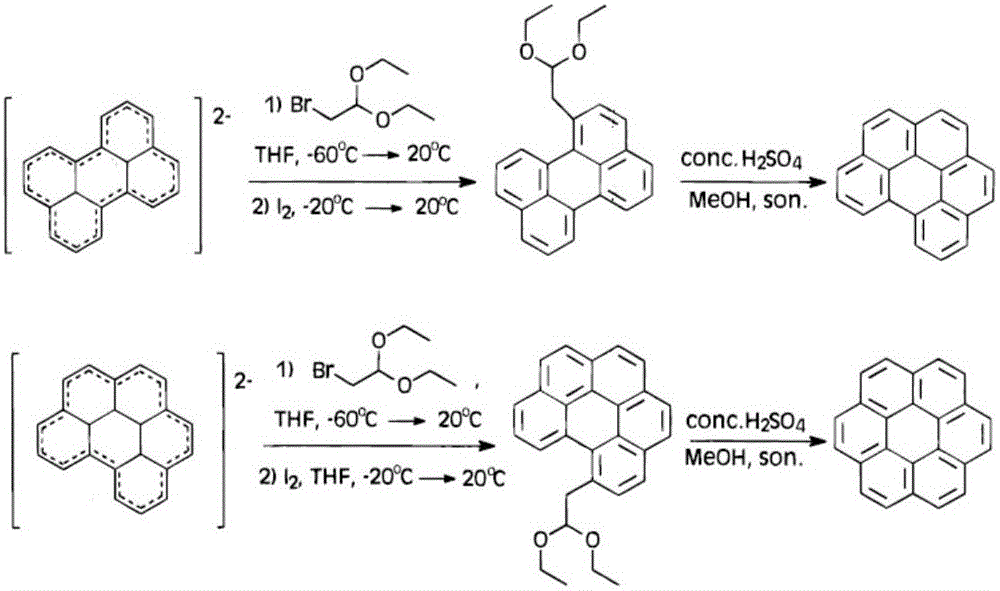
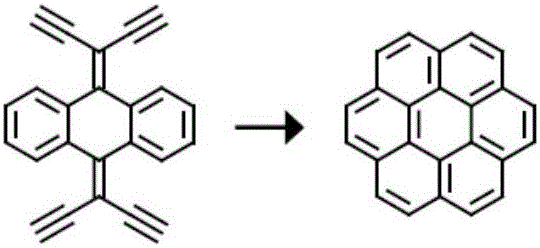
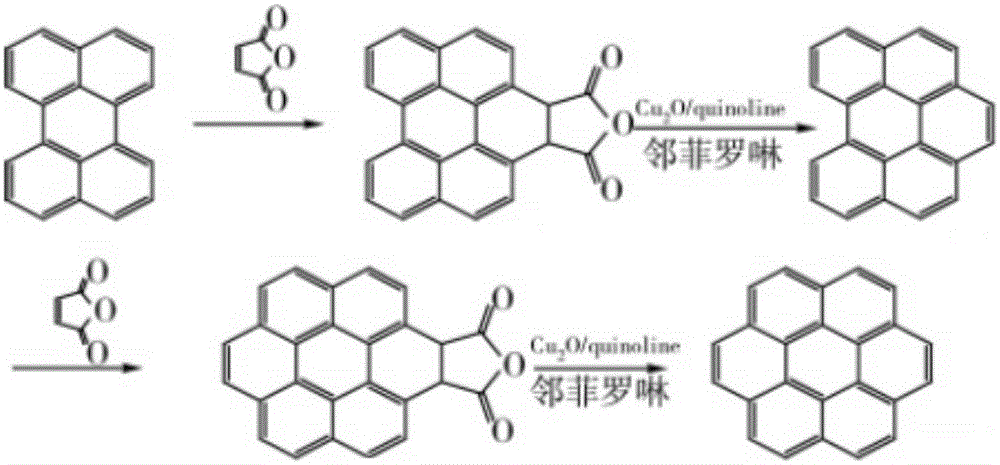
[0035] The Chinese alias of corona benzene is Kou, and its molecular formula is C 24 h 12 , also known as hexabenzocene, is a polycyclic aromatic hydrocarbon with a highly symmetrical structure composed of six benzene rings fused around it; coronene exhibits blue-purple fluorescence in organic solvents.
[0036] In the preparation method of coronene of the present invention, the preparation method a...
Embodiment approach
[0043] According to one embodiment of the present invention, the amount of methanesulfonic acid or trifluoromethanesulfonic acid is 5-18 equivalents.
[0044] According to the preparation method of coronene of the present invention, when the amount of methanesulfonic acid or trifluoromethanesulfonic acid adopts the above-mentioned amount, the amount of by-products produced by the reaction will be reduced to the minimum, the purity of the product will be increased, and the product yield will be improved.
[0045] According to one embodiment of the present invention, the ring-closing step is reacted in dichloromethane solvent.
[0046] According to the preparation method of coronene of the present invention, in the substitution reaction, because the solubility of the compound shown in the formula III is good in methylene chloride, the compound shown in the formula III can be fully reacted and the product yield can be improved.
[0047] According to one embodiment of the present ...
Embodiment 1
[0054] The synthetic route is as follows:
[0055]
[0056] According to the preparation method of coronene of the present invention, first enter coupling reaction, under the protection of nitrogen, 5.2g (0.01mol, 1eq) compound shown in formula I (prepared according to Eur.J.Org.Chem.2008,994-1004) , 8.71g (0.044mol, 4.4eq) compound shown in formula II (prepared according to WO2014 / 37750), 0.46g (0.0004mol, 0.04eq) tetrakistriphenylphosphine palladium, 12.1g (0.088mol, 8.8eq) potassium carbonate , 80 ml of toluene, 60 ml of ethanol, add to the reactor and stir evenly, slowly raise the temperature to 60-70 ° C for 8 hours, then lower it to 20 ° C, add 100 ml of water, 100 ml of toluene, stir, separate liquid, toluene layer Washed twice with water, dried over anhydrous sodium sulfate, filtered to remove the desiccant, then passed through a silica gel column, the eluate was concentrated to dryness under reduced pressure, and 80 ml of dichloromethane was added to obtain a dichl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com