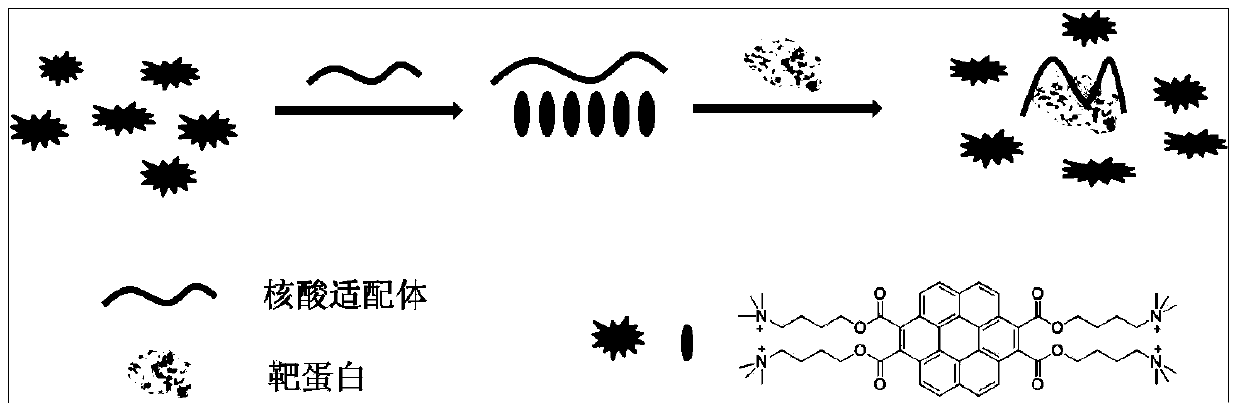
Coronene derivative probe and preparation method thereof, and protein detection method based on coronene derivative probe and aptamer
A nucleic acid aptamer and protein detection technology, applied in the field of preparation, hexacene derivative probes, and protein detection based on hexacene derivative probes and nucleic acid aptamers, can solve the problem of high cost and sensitivity Low, cumbersome steps and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The present invention also provides a preparation method of a hexabenzocene derivative probe, comprising:
[0038] Step 1: Add perylene, N-ethylmaleimide, tetrachlorobenzoquinone and p-methoxyphenol in the reaction vessel, and react at 230-280°C for 4-12 hours under the protection of nitrogen, and react to In the later stage, the solution becomes viscous, and then dimethylformamide is added, reacted for 30-60 minutes, filtered, washed, and dried to obtain orange powdery solid compound 1, and the molar ratio of perylene and p-methoxyphenol is preferably 2 : 1, the mol ratio of described perylene, N-ethylmaleimide, chloranil and p-methoxyphenol is preferably (2.4-4.8): (10-80): (4-20) : (1.2-2.4), the amount of the solvent dimethylformamide is not particularly limited, as long as it can dissolve the reaction mixture;
[0039] Step 2: Mix compound 1, potassium hydroxide and isopropanol obtained in step 1, react at 85-100°C for 12-24 hours, add excess water, adjust pH to 3...
Embodiment 1
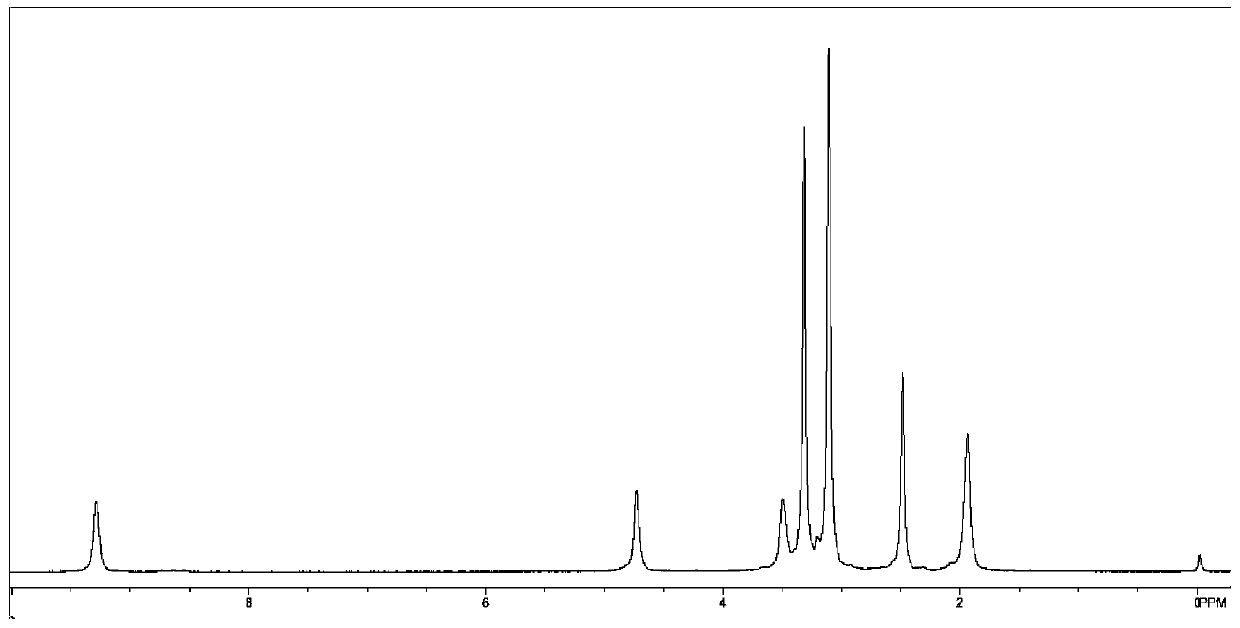
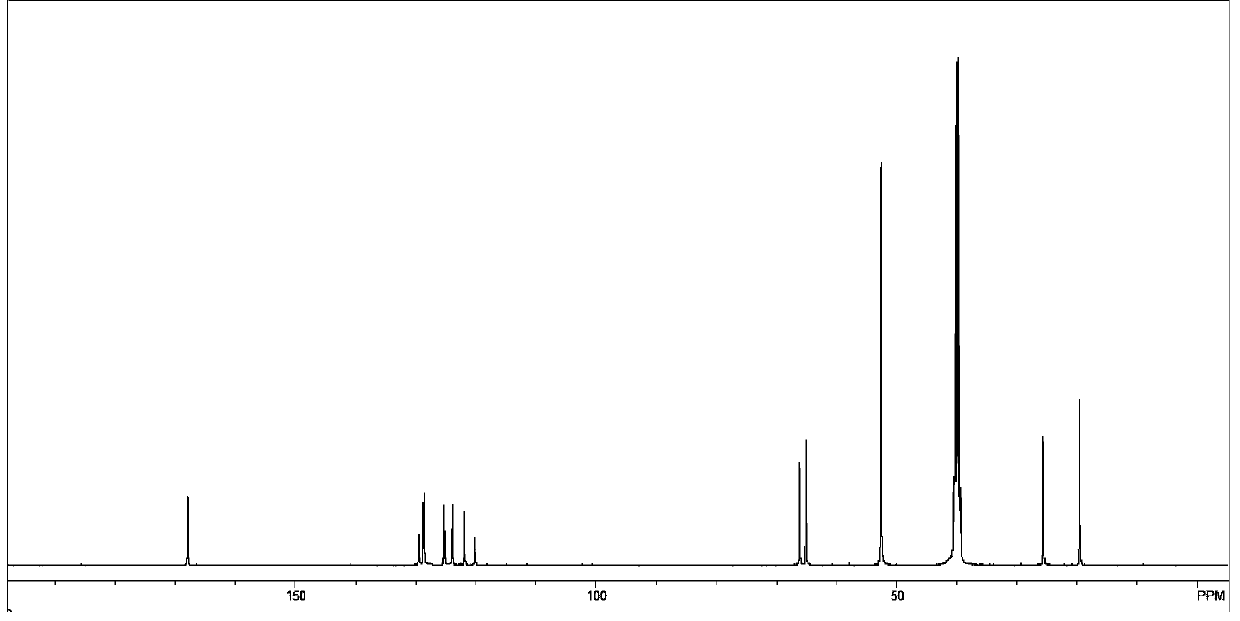
[0051] Step 1: Add 2.4mmol perylene, 40mmol N-ethylmaleimide, 9mmol tetrachlorobenzoquinone, 1.2mmol p-methoxyphenol to a 50mL single-necked bottle, and react at 250°C for 6h under nitrogen protection, then add 30mL di Methylformamide was refluxed for 30 minutes, filtered, washed with methanol and ether, and dried to obtain an orange powdery solid compound 1;
[0052] Step 2: Add 0.2mmol compound 1, 0.3gKOH, and 30mL isopropanol to a 100mL single-necked bottle, react at 95°C for 12 hours, add 30mL water, add dilute hydrochloric acid to adjust the pH to 4, filter, wash with water and ethanol, and vacuum dry , to obtain red solid compound 2;
[0053] Step 3: Add 0.5mmol of compound 2, 1gKOH, and 30mL of water into a 100mL two-necked flask, react at 90°C for 2h, cool to room temperature, adjust the pH to 8, add 0.2g of tetra-n-octylammonium bromide and 2mL of 1,4-dibromide Butane, react at 130°C for 3 hours, cool down, add 30mL chloroform to the reaction bottle, wash the organic...
Embodiment 2
[0060] Step 1: Add 4.8mmol perylene, 80mmol N-ethylmaleimide, 20mmol tetrachlorobenzoquinone, 2.4mmol p-methoxyphenol to a 100mL single-necked bottle, react at 230°C for 4h, then add 60mL Dimethylformamide was refluxed for 60 minutes, filtered, washed with methanol and ether, and dried to obtain orange powdery solid compound 1;
[0061] Step 2: Add 0.2mmol compound 1, 0.6gKOH, and 30mL isopropanol to a 100mL single-necked bottle, react at 85°C for 24 hours, add 50mL water, add dilute hydrochloric acid to adjust the pH to 4, filter, wash with water and ethanol, and vacuum dry , to obtain red solid compound 2;
[0062] Step 3: Add 0.5mmol of compound 2, 1gKOH, and 30mL of water into a 100mL two-neck flask, react at 85°C for 6h, cool to room temperature, adjust the pH to 8, add 0.2g of tetra-n-octylammonium bromide and 2mL of 1,4-dibromide Butane, react at 100°C for 6 hours, cool, add 40mL chloroform to the reaction bottle, wash the organic layer with water and saturated NaCl aq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com