A method for preparing coronene compounds from alcohol raw materials
A technology for compounds and alcohols, applied in the field of preparing coronene compounds, can solve the problems of unstable intermediate products, inconvenient industrialization and the like, and achieve the effects of being suitable for large-scale production, low cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Synthesis of RHO molecular sieve catalyst
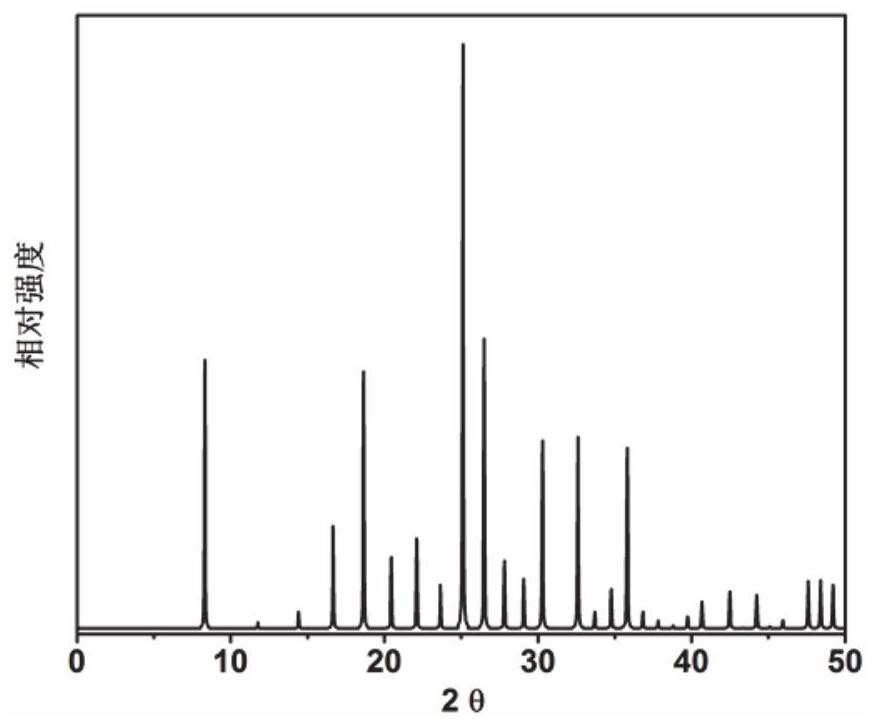
[0067] Initial gel molar composition ratio 2N-methylbutylamine (as organic structure directing agent): 3Na 2 O: 0.4Cs 2 O: Al 2 O 3 : 10SiO 2 : 110H 2 O Mix the metered amounts of sodium oxide, cesium oxide, pseudoboehmite, silica sol and deionized water in a beaker, stir well to form a gel, and then put it into a stainless steel autoclave lined with Teflon, in 100℃ constant temperature for 50h. After crystallization, centrifuge at 3000 rpm / centrifugation for 3 min, and the solid product obtained by separation is washed with deionized water to neutrality, and dried in air at 120 °C overnight. The results of XRD analysis are as follows: figure 1 shown. from figure 1 It can be seen from the results that the synthesized product is the original powder of RHO molecular sieve, which is calcined at 550°C for 5 hours to obtain RHO molecular sieve, which is denoted as catalyst 1.
Embodiment 2
[0068] Example 2: Synthesis of ITQ-29 molecular sieve catalyst
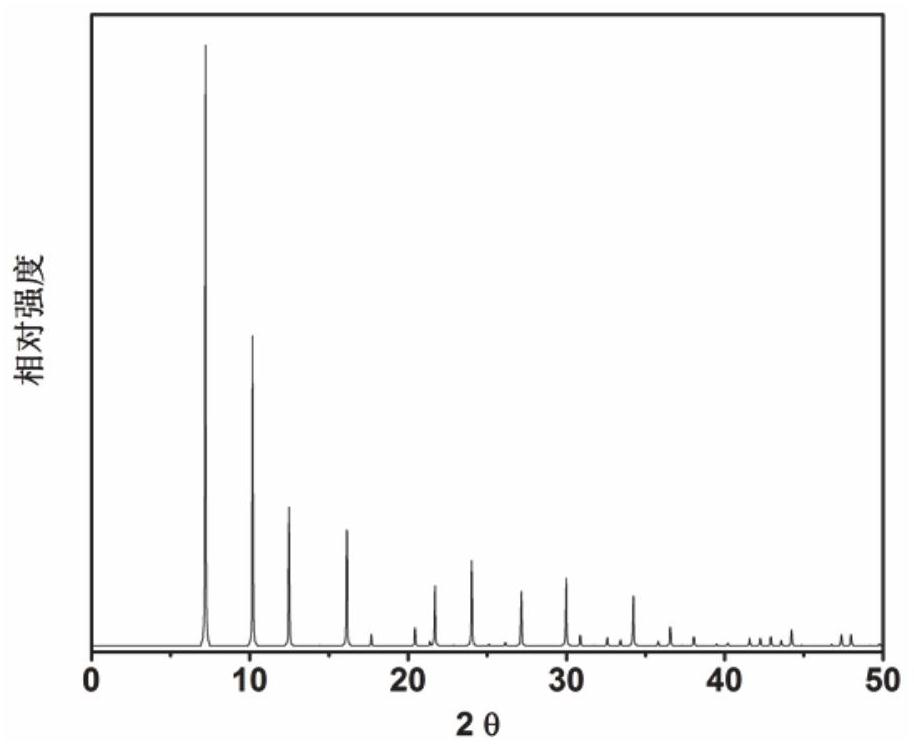
[0069] In the initial gel molar composition ratio of 1.85 2,2-dimethyl-2,3-dihydro-1H-phenyl[de]isoquinoline (as an organic structure directing agent): 0.2SiO 2 : 0.8GeO 2 : 0.06Al 2 O 3 : 0.25 4-methyl-2,3,6,7-tetrahydro-1hydro, 5hydro-pyridine[3,2,1-ij]quinoline: 0.25 tetramethylamine: 0.5HF: 6H 2 O Mix measured amounts of silica sol, germanium dioxide, aluminum isopropoxide, organic structure directing agent, hydrofluoric acid, and deionized water in a beaker, stir well to form a gel, and then load into a Teflon-lined In a stainless steel autoclave, 6d was crystallized at a constant temperature of 135 °C. After crystallization, centrifuge at 3000 rpm / centrifugation for 3min, and the solid product obtained by separation is washed with deionized water to neutrality, and after drying in air at 100 °C overnight, the results of XRD analysis are as follows: figure 2 shown. from figure 2 It can be seen from ...
Embodiment 3
[0070] Example 3: Synthesis of UZM-9 molecular sieve catalyst
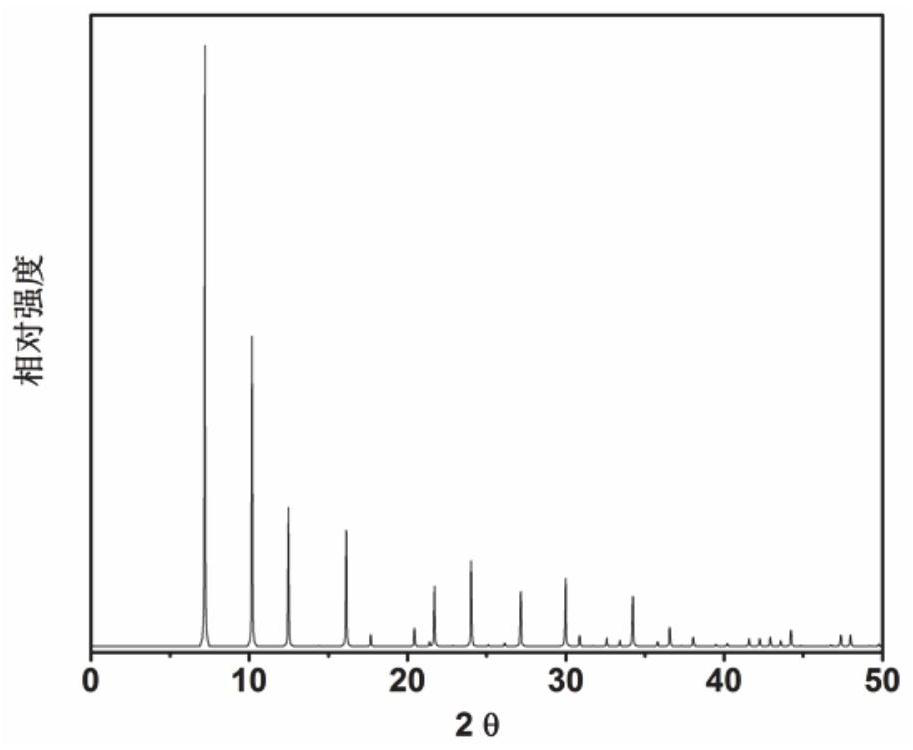
[0071] In the initial gel molar composition ratio 9 tetraethyl ammonium hydroxide: 2 tetramethyl ammonium hydroxide: 0 Na 2 O: Al 2 O 3 : 16SiO 2 : 62H 2 O Mix the measured amounts of tetraethylammonium hydroxide, tetramethylammonium hydroxide (organic structure directing agent), aluminum isopropoxide, silica sol, and deionized water in a beaker, stir well to form a gel, and then load into a In a stainless steel autoclave lined with Teflon, it was crystallized at room temperature for 6 h. After crystallization, centrifuge at 3000 rpm / centrifugation for 3min, and the solid product obtained by separation is washed with deionized water to neutrality, and after drying in air at 100 °C overnight, the results of XRD analysis are as follows: image 3 shown. from image 3 It can be seen from the results that the synthesized product is the original powder of UZM-9 molecular sieve, which is calcined at 600 °C for 5 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com