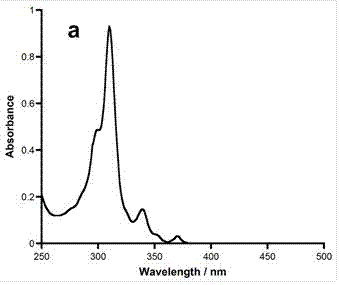
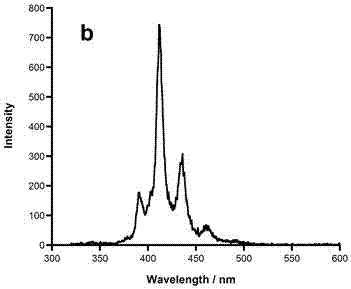
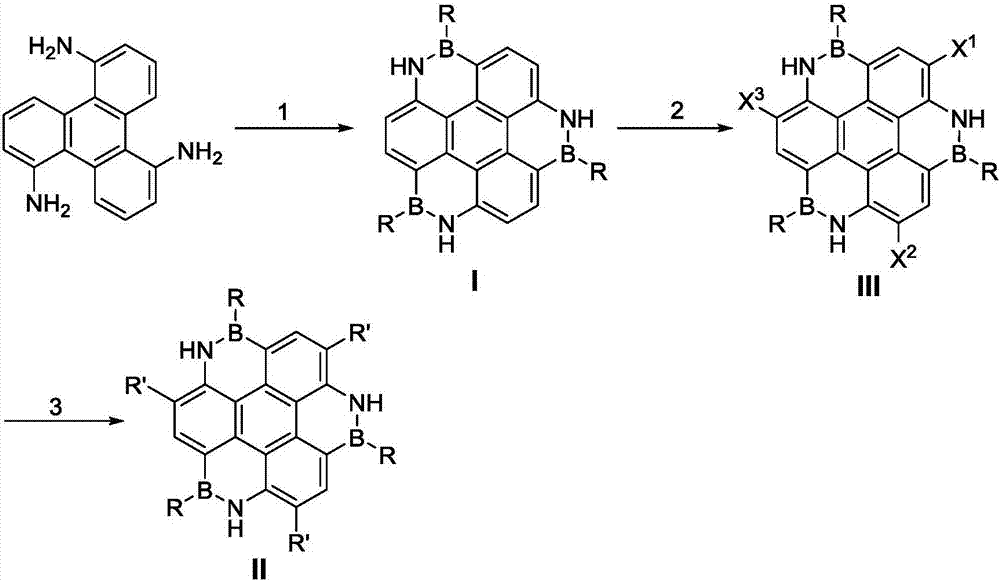
Boron-nitride doped coronene compound and preparation method thereof
A compound and boron nitrogen technology, which is applied in the field of polycyclic aromatic hydrocarbon compounds and their preparation, can solve the problems of inability to synthesize alkoxy-free boron nitride compounds, unfavorable derivative expansion of conjugated systems, limited application and the like, and achieves short synthesis steps. , easy to operate, less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: 1,5,9-Triaza-2,6,10-triphenylboronidine
[0022]
[0023] 218.7 mg of triamine-based compound, the preparation method is referred to Chem. Commun. 2016, 52, 537-540, dissolved in o-dichlorobenzene (22 mL), 508.2 mg of phenyldichloroboron, 485.7 mg of triethylamine were added under nitrogen, and refluxed 24h. After the reaction, the o-dichlorobenzene was evaporated under reduced pressure, and then the solvent was evaporated. The mixed solvent with a volume ratio of petroleum ether and dichloromethane of 1:1 was used as the eluent to separate by silica gel column chromatography to obtain a white solid compound 1,5. ,9-triazide-2,6,10-triphenylboronidine 261mg, the yield is 61%, melting point: >300℃.
[0024] The obtained 1,5,9-triaza-2,6,10-triphenylboronidine was characterized as follows: IR (KBr, cm -1 ): 3385, 3044, 1599, 1484, 1422, 1328, 1247, 700; 1 H NMR (500MHz, CDCl 3 ):δ8.77(d,J=8.0Hz,3H),8.42(s,3H),8.03(dd,J=7.8Hz,1.5Hz,6H),7.86(d,J=8.0Hz,3H),...
Embodiment 2
[0025] Example 2: 1,5,9-Triaza-2,6,10-tris(4-methylphenyl)boronidine
[0026]
[0027] Adopt a synthetic method similar to Example 1, replace phenylboron dichloride with equimolar 4-methylphenylboron dichloride (refer to J.Org.Chem.2000,65,9125-9128 for preparation method), The ratio of the eluent was adjusted to 2:1, and other steps were the same as in Example 1 to obtain a white solid compound 1,5,9-triaza-2,6,10-tris(4-methylphenyl)boronidine 258.2mg , its yield is 56%, melting point: >300 ℃.
[0028] The obtained 1,5,9-triaza-2,6,10-tris(4-methylphenyl)boronidine was characterized as follows: IR(KBr,cm -1 ): 3741, 3385, 3008, 1599, 1422, 1324, 815; 1 H NMR (500MHz, CDCl 3 )δ8.70(d, J=7.9Hz, 3H), 8.27(s, 3H), 7.91(d, J=7.5Hz, 6H), 7.75(d, J=8.0Hz, 3H), 7.44(d, J=7.4Hz, 6H), 2.54(s, 9H); 13C NMR (125 MHz, CDCl 3 )δ141.0,138.8,136.7,135.4,134.5,133.9,129.2,123.9,116.9,115.8,21.8; 11 B NMR (160MHz, CDCl 3 ):δ35.9; HRMS(DART Positive)m / z calcd forC 39 H 31 10 B 3...
Embodiment 3
[0029] Example 3: 1,5,9-Triaza-2,6,10-tris(4-trifluoromethylphenyl)boronidine
[0030]
[0031] The synthesis method similar to Example 1 was adopted, and equimolar 4-trifluoromethylphenylboron dichloride was used for phenylboron dichloride (refer to J.Org.Chem. 2000, 65, 9125-9128 for the preparation method) Replacement, other steps are the same as in Example 1, to obtain a pale yellow solid compound 1,5,9-triazide-2,6,10-tris(4-trifluoromethylphenyl)boronidine 352.2mg, its yield is 54%, melting point: >300 ℃.
[0032] The obtained 1,5,9-triazide-2,6,10-tris(4-trifluoromethylphenyl)boronamidine was characterized as follows: IR (KBr, cm -1 ): 3402, 3038, 1599, 1327, 1118, 1063, 828, 695; 1 H NMR (500MHz, CD 3 COCD 3 ): δ9.68(s,3H),8.28(d,J=7.9Hz,3H),8.02(d,J=7.4Hz,6H),7.84(d,J=7.9Hz,3H),7.80(d ,J=7.5Hz,6H); 19 F NMR (470MHz, CD 3 COCD 3 ):δ-62.9(s,CF 3 ); 13 C NMR (125MHz, CD 3 COCD 3 ): δ145.6,141.7,135.6, 134.9,134.2,130.5(q, 2 J C-F =31.6Hz), 125.8(q, 1 J ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com