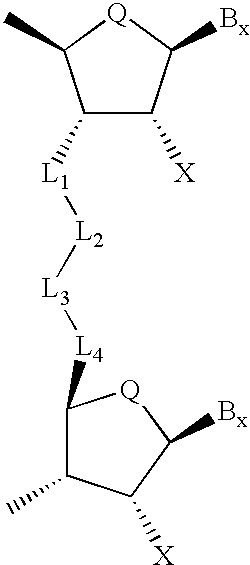Patents
Literature
55results about How to "Sequencing is facilitated" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
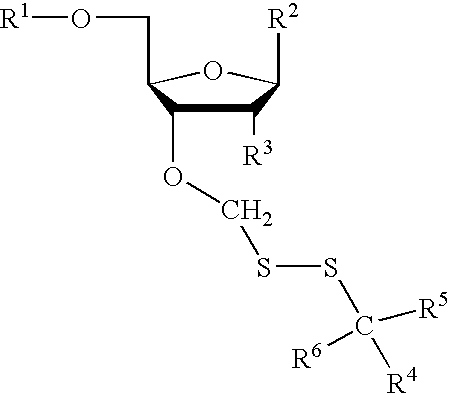
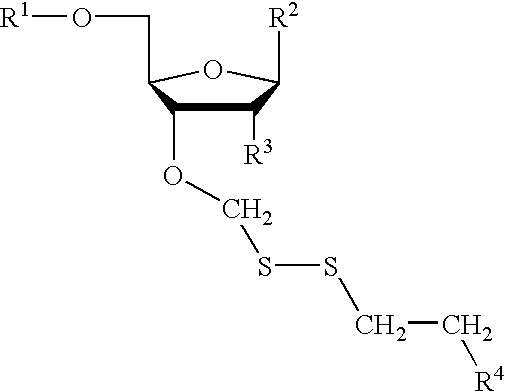
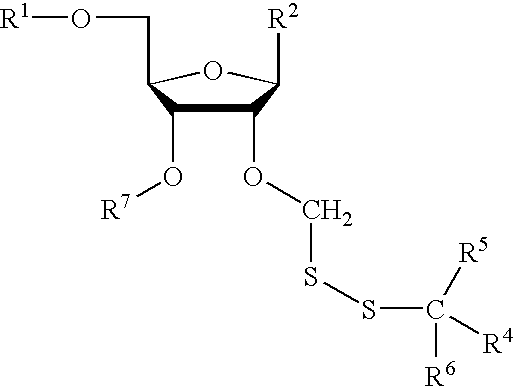
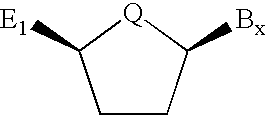
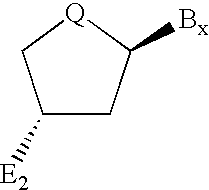
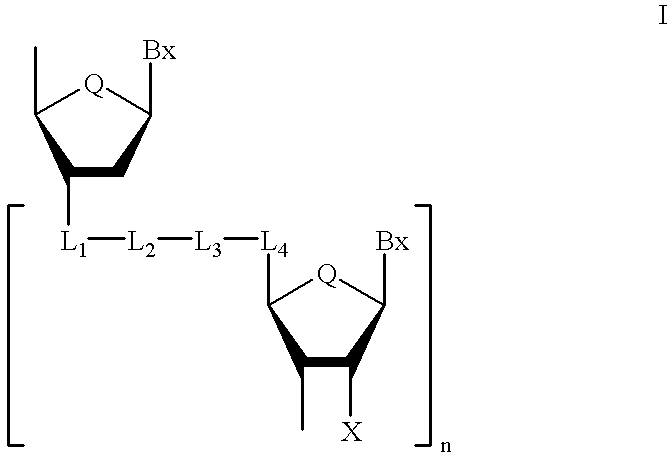
Compounds for protecting hydroxyls and methods for their use
InactiveUS7279563B2Sequencing is facilitatedEasy to implementSugar derivativesOrganic compound preparationChemical synthesisNucleotide
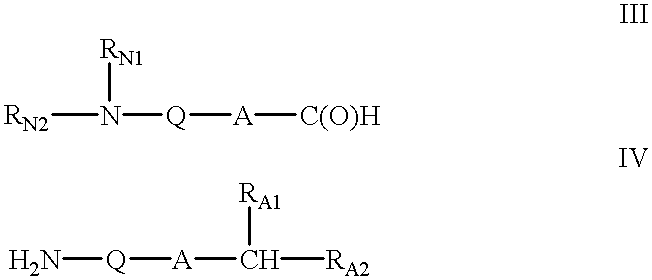
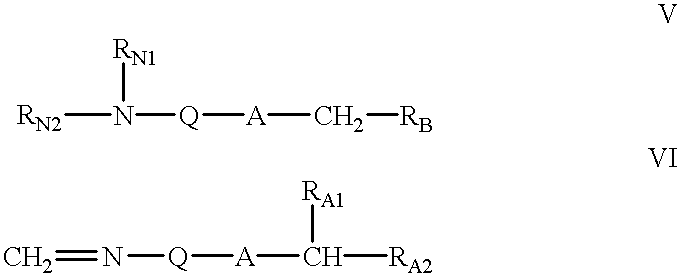
A hydrocarbyldithiomethyl-modified compound of the Formula:R1—O—CH2—S—S—R2or a salt thereof wherein R1 is an organic molecule and R2 is a hydrocarbyl is useful for protecting and / or blocking hydroxyl groups in organic molecules such as nucleotides. The hydrocarbyldithiomethyl-modified compounds can also be used for chemically synthesizing oligonucleotides and for sequencing nucleic acid compounds.
Owner:FLUIDIGM CORP
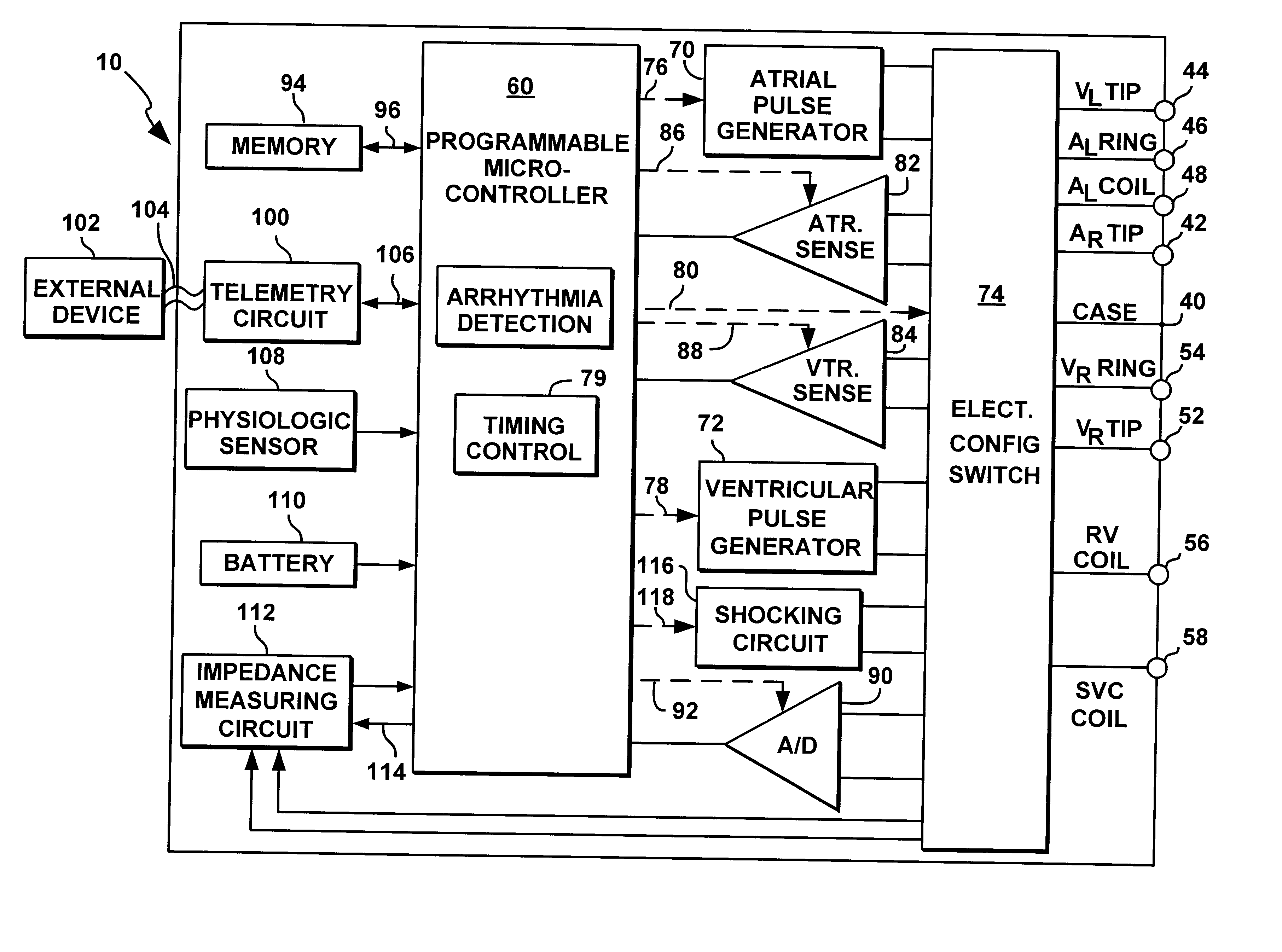
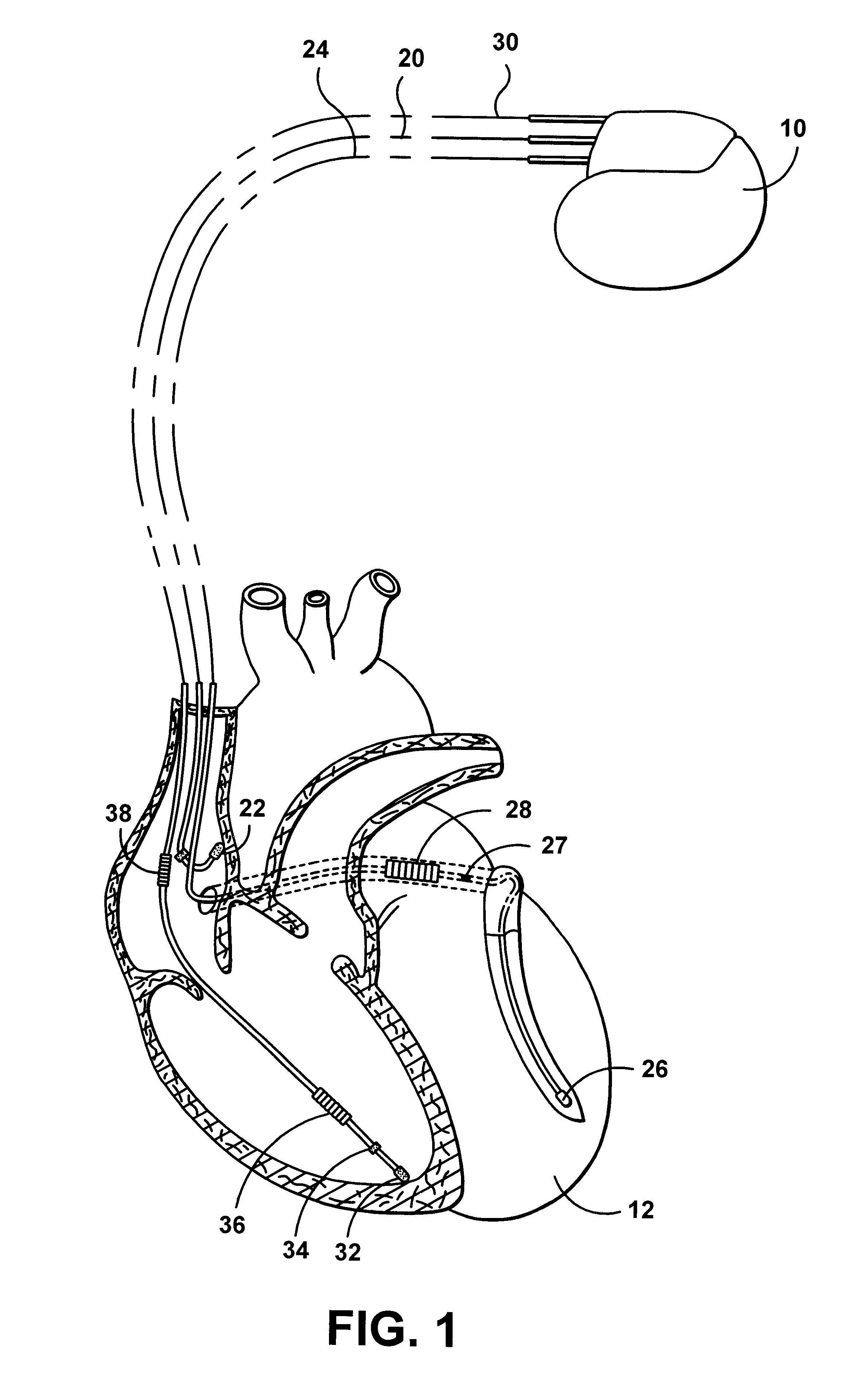
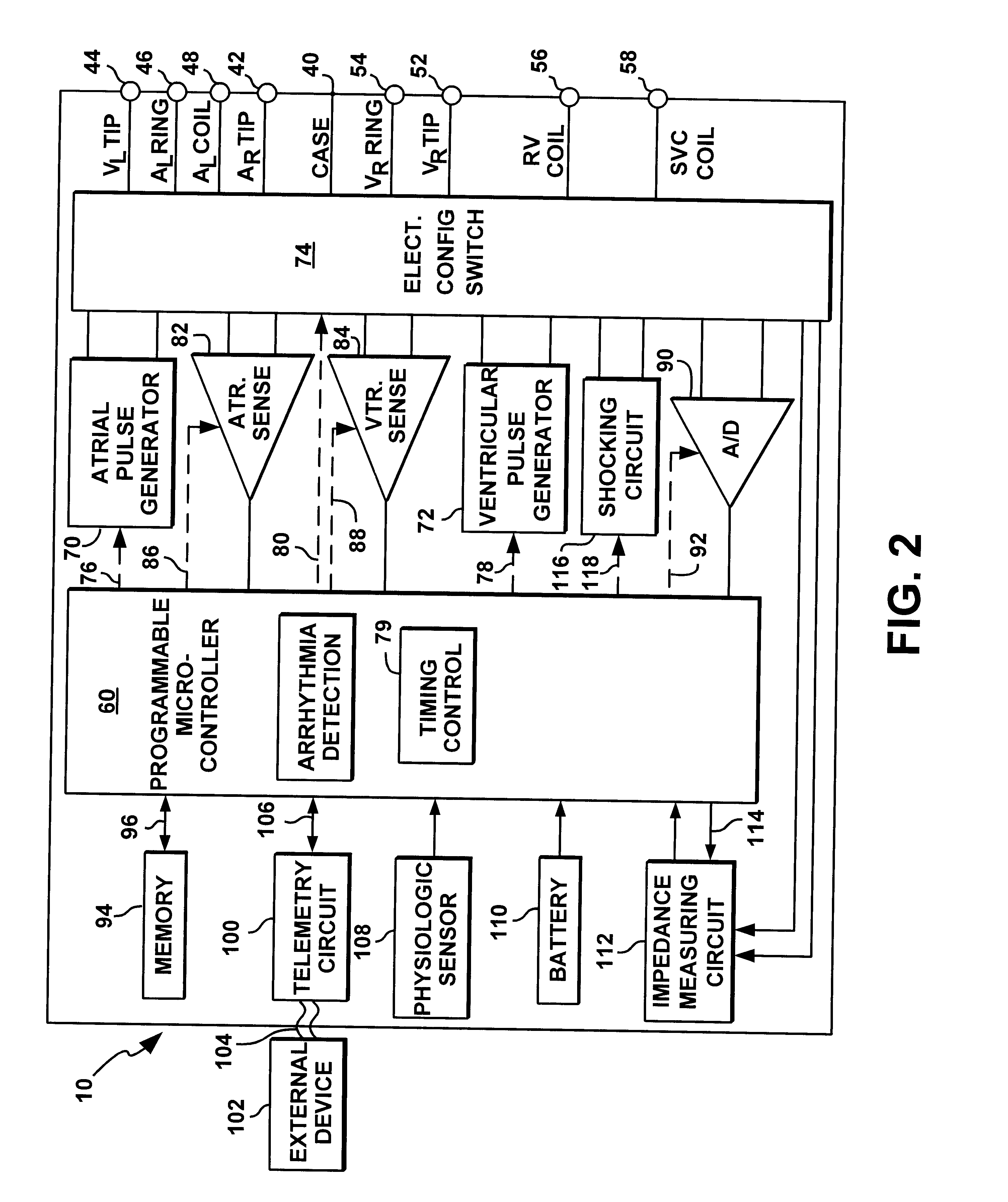
System and method for automatically selecting electrode polarity during sensing and stimulation
InactiveUS6477417B1Improve welfareImprove coordinationHeart defibrillatorsHeart stimulatorsElectricityElectrical battery
An implantable multi-chamber cardiac stimulation device includes flexibly programmable electrode stimulation configurations, and is capable of precisely controlling the stimulation sequence between multiple sites. The stimulation device provides a plurality of connection ports that allow independent connection of each electrical lead associated with a particular stimulation site in the heart. Each connection port further provides a unique terminal for making electrical contact with only one electrode such that no two electrodes are required to be electrically coupled. Furthermore, each electrode, whether residing on a unipolar, bipolar or multipolar lead, may be selectively connected or disconnected through programmable switching circuitry that determines the electrode configurations to be used for sensing and for stimulating at each stimulation site. The stimulation device allows for the programmable selection of each electrode terminal connection to a relatively positive or negative battery potential. In this way, each electrode, when electrically connected, may be programmed to act as the cathode or as the anode during sensing or stimulation delivery. Thus, directionality of the depolarization wave may be controlled by programming the cathode and anode assignments of the stimulation electrodes.
Owner:PACESETTER INC
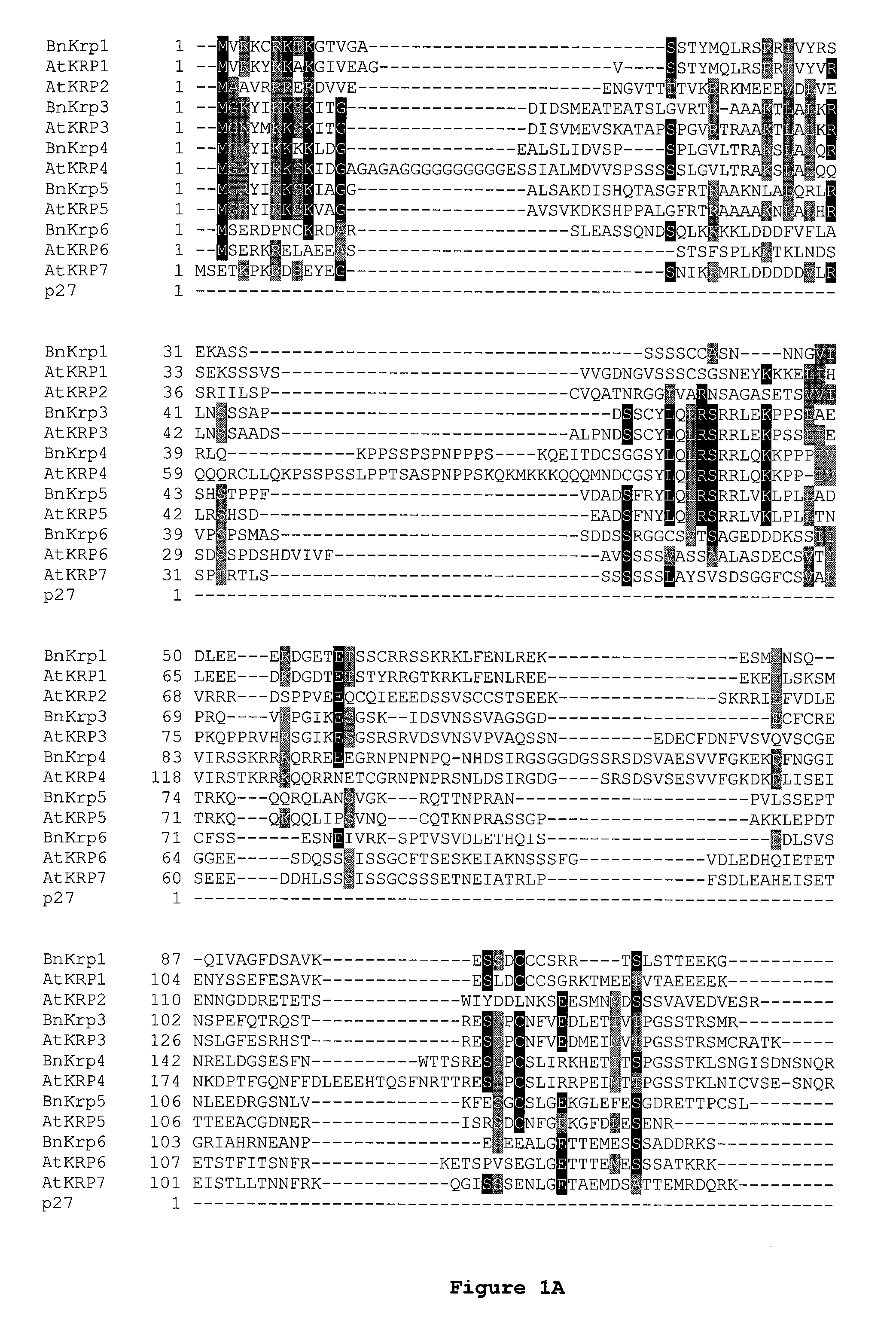
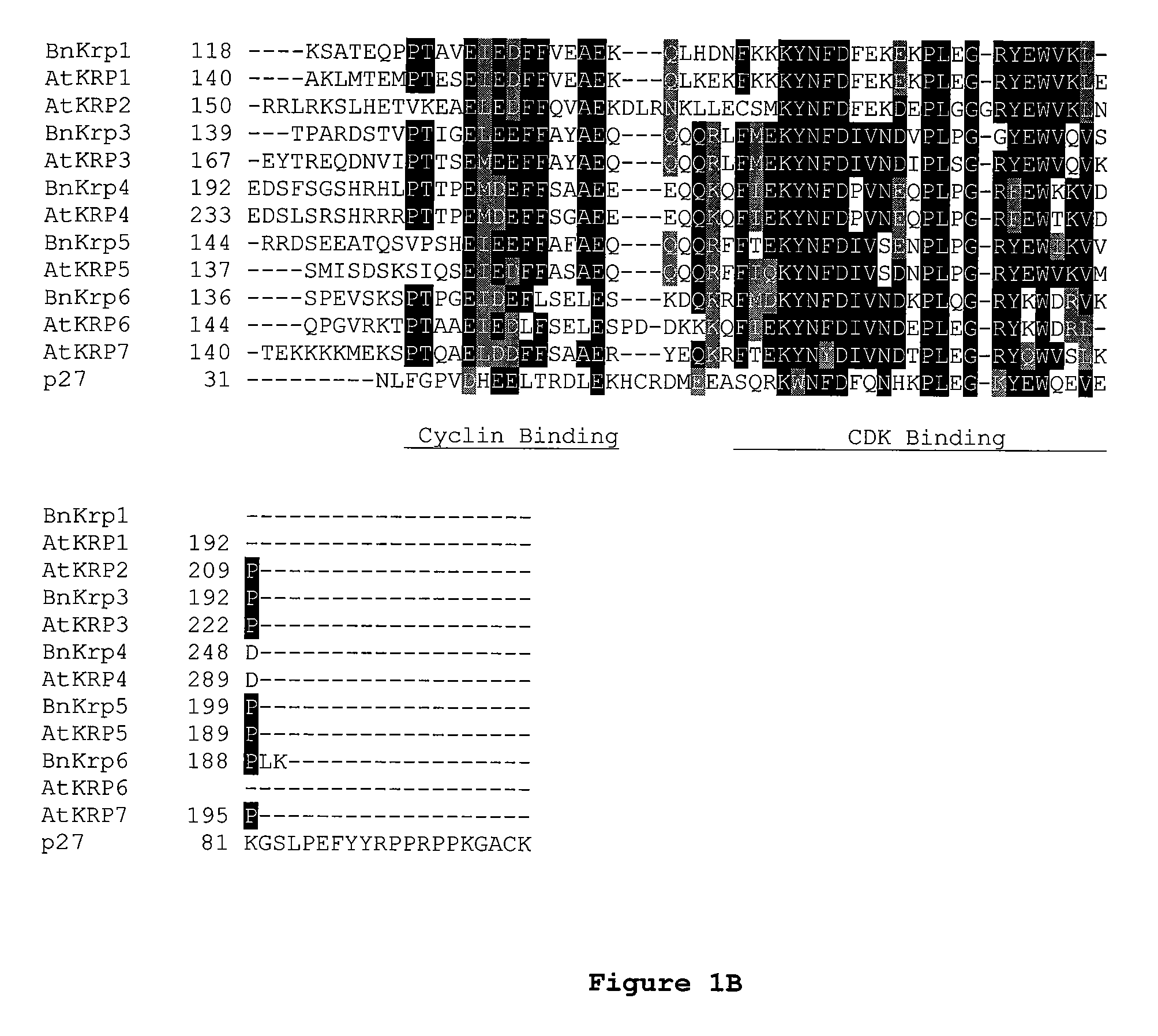
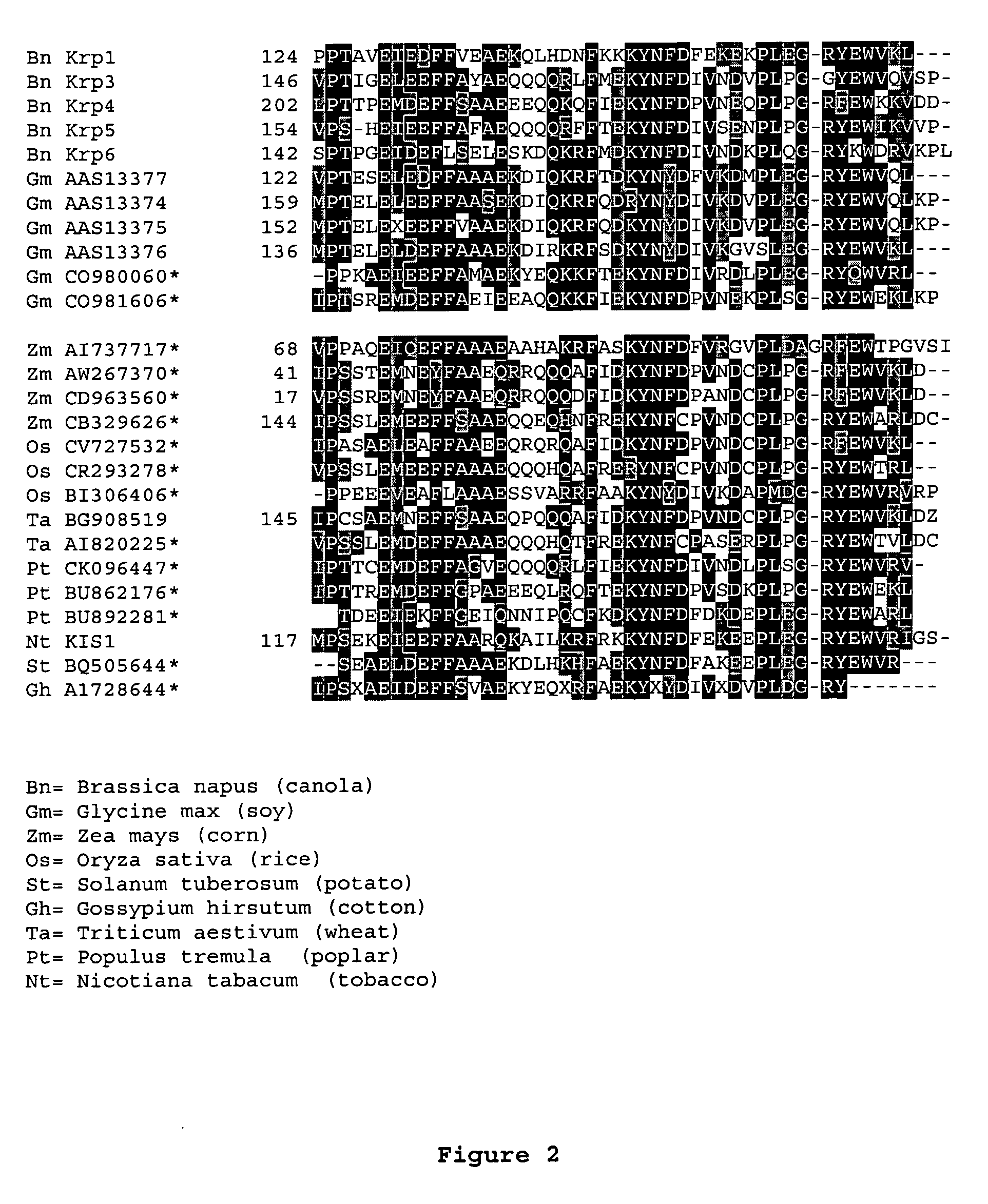
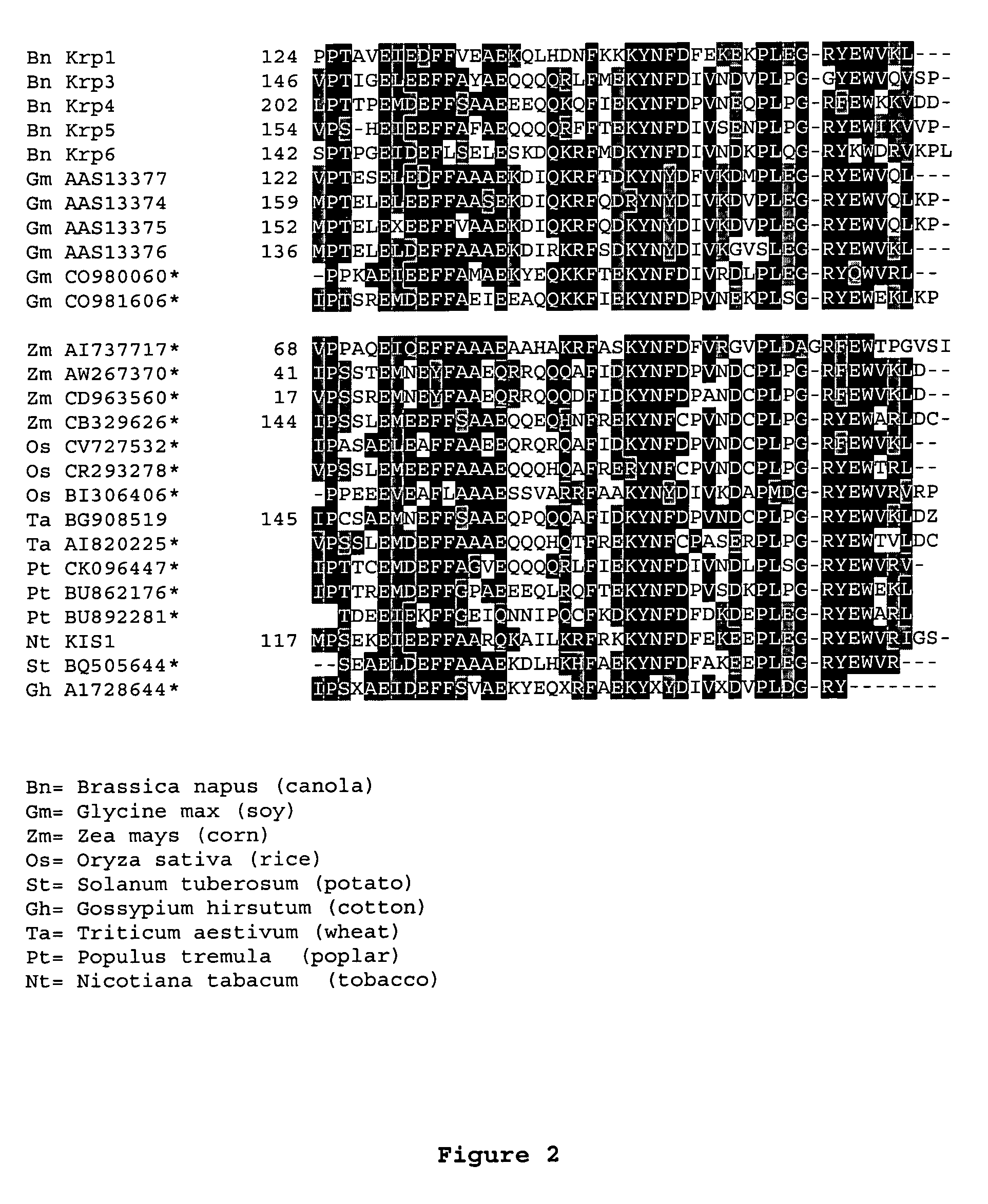
Dominant negative mutant krp protein protection of active cyclin-cdk complex inhibition by wild-type krp
InactiveUS20070056058A1Speed upIncreasing cell proliferationBacteriaSugar derivativesMutated proteinPlant cell
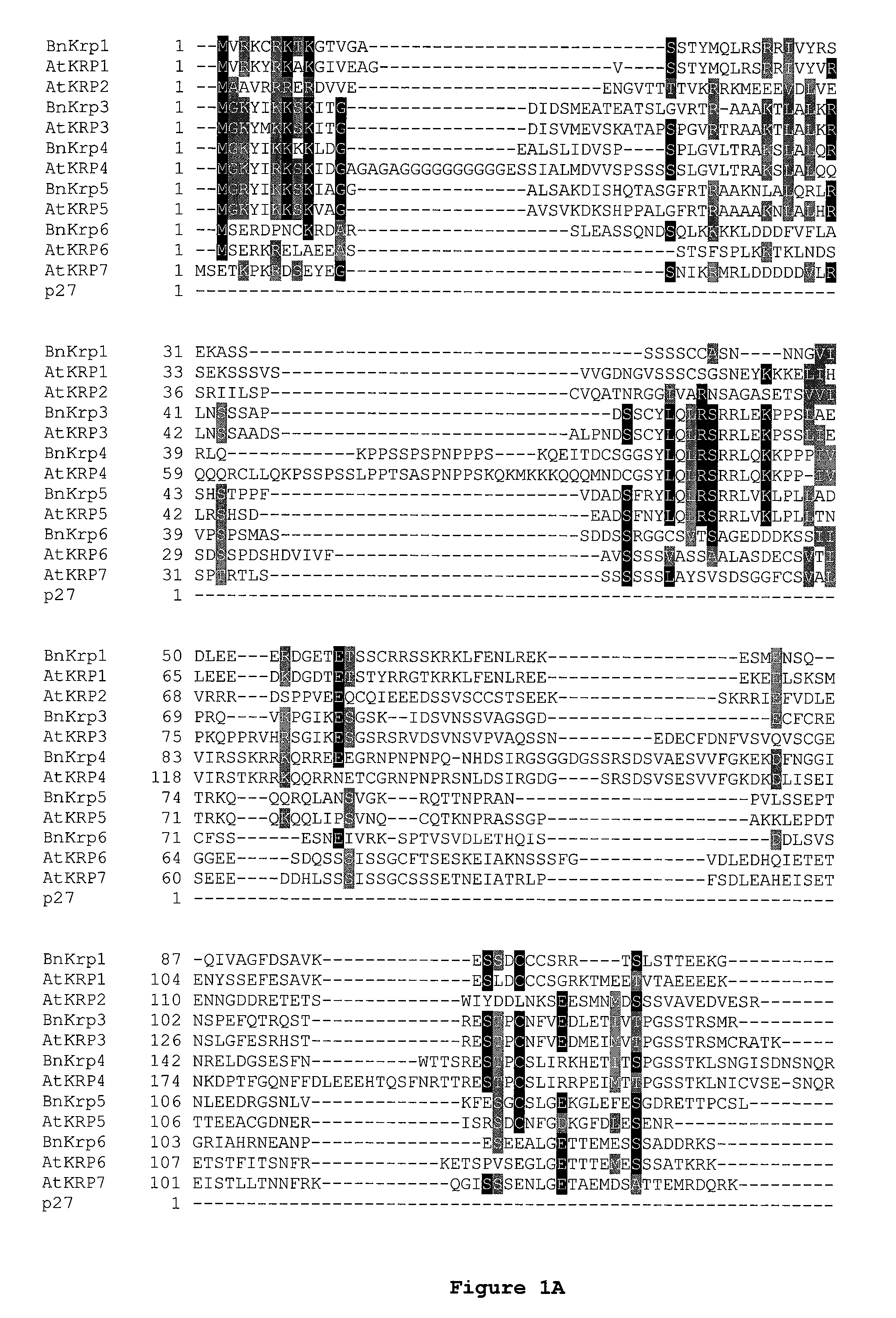
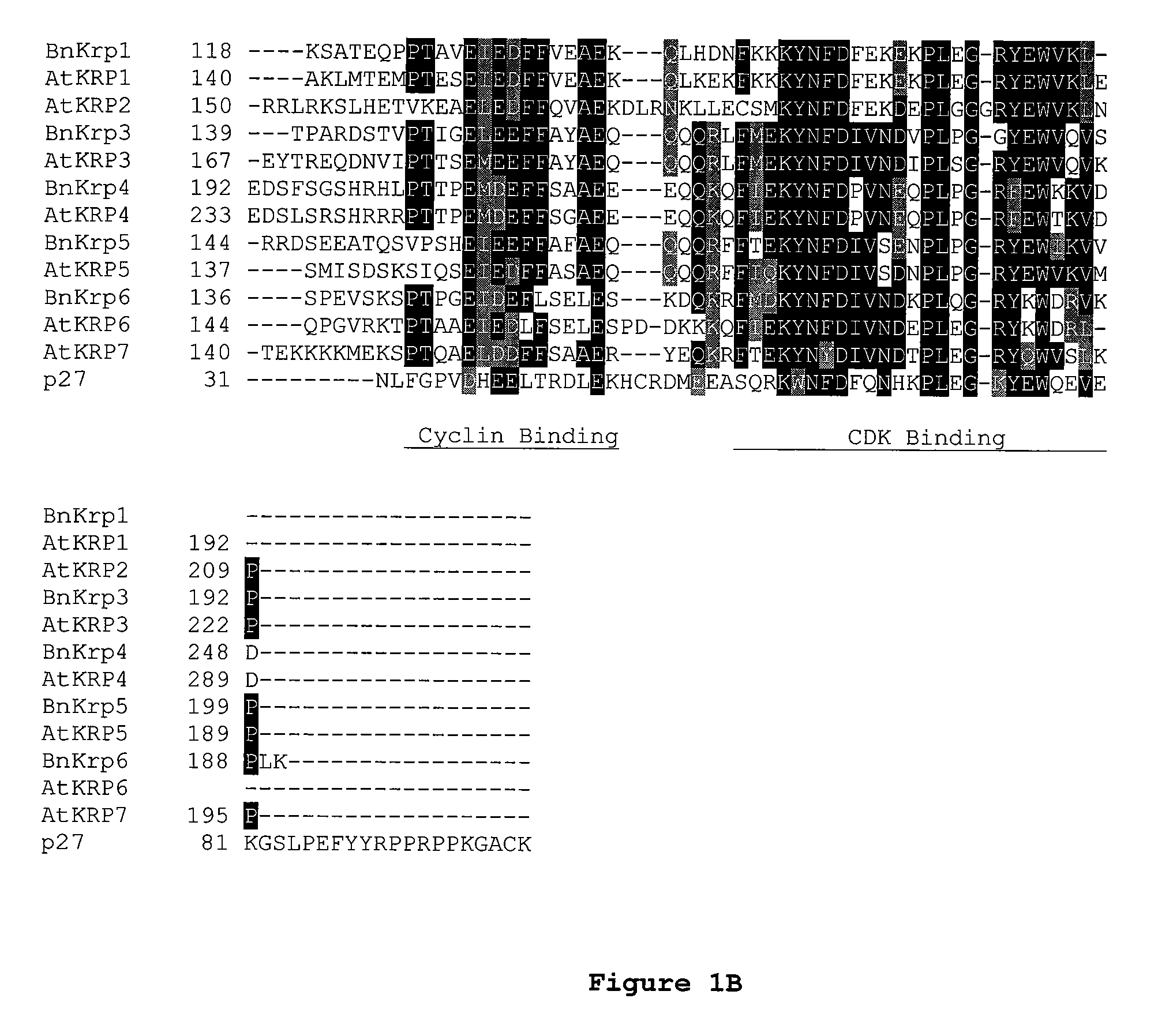
Disclosed are mutant CDK inhibitor (CKI) polypeptides having dominant negative antagonist activity against wild-type CKI proteins, as well as related compositions, including nucleic acids and vectors encoding the mutant CKI polypeptides and transformed host cells and transgenic plants comprising such nucleic acids and vectors. Also disclosed are related methods for using the mutant proteins to modulate cell division in cells, particularly plant cells.
Owner:TARGETED GROWTH
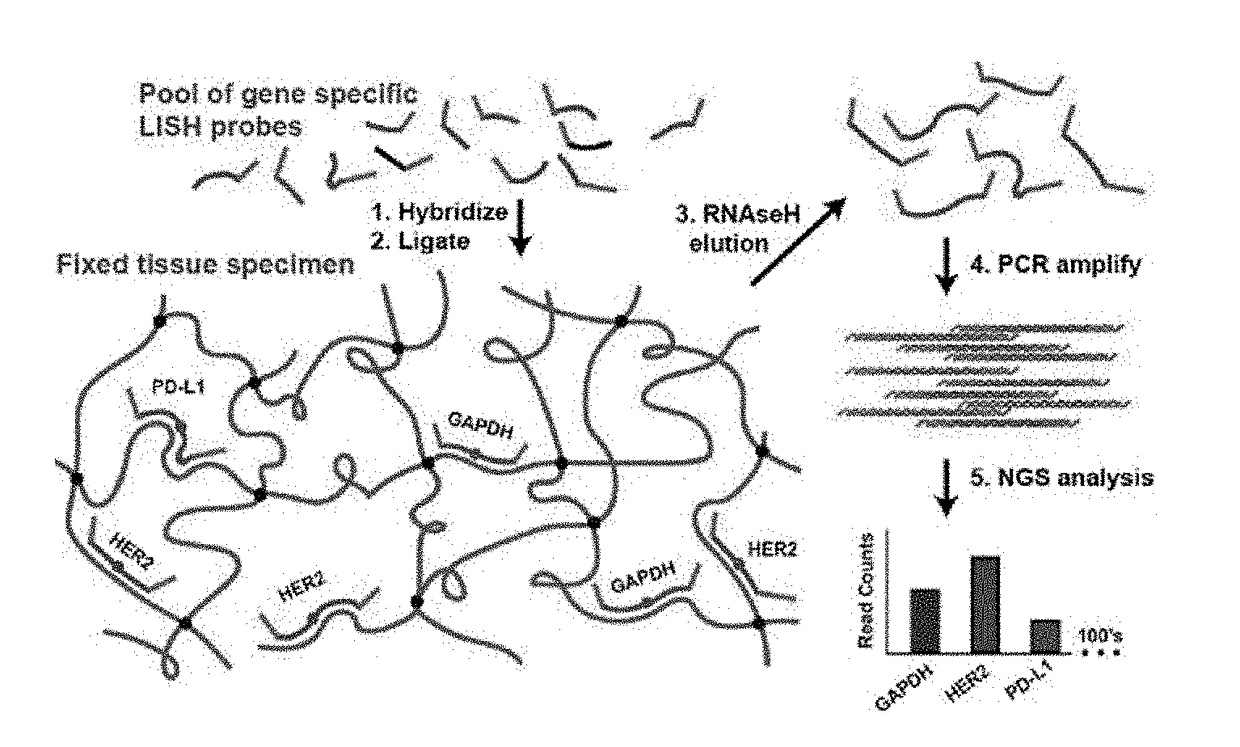
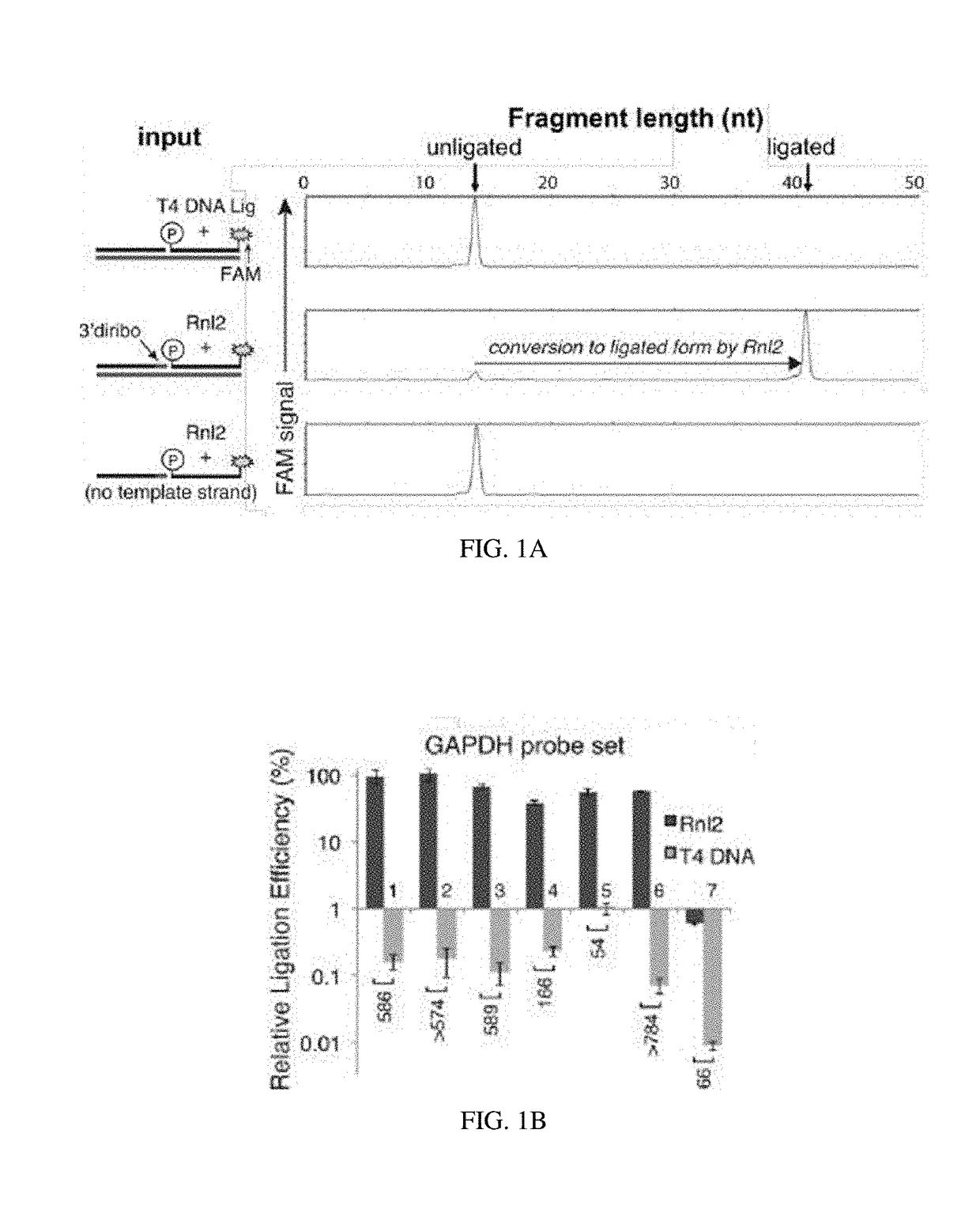
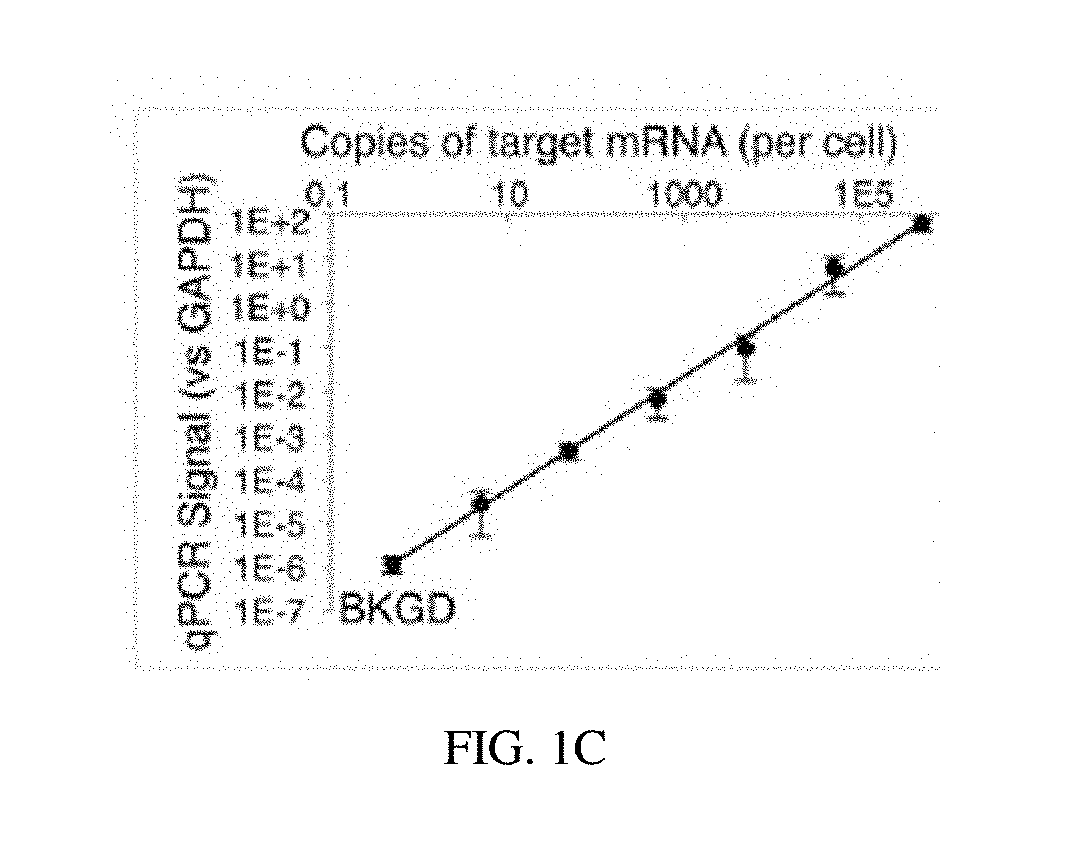
Compositions and methods of RNA analysis
PendingUS20180208967A1Reduce probabilityIncrease volumeMicrobiological testing/measurementRNA analysisMolecular probe
The present disclosure relates to compositions and methods of RNA analysis. In particular, the present disclosure provides a method of RNA analysis that includes obtaining a sample, applying one or more multi-partite probes to the sample, where each of the one or more multi-partite probes includes at least two sub-probes, annealing at least one of the applied one or more multi-partite probes to at least one target nucleic acid within the sample, and ligating the at least two sub-probes associated with the at least one annealed multi-partite probe to create a target nucleic acid proxy that can be detected.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
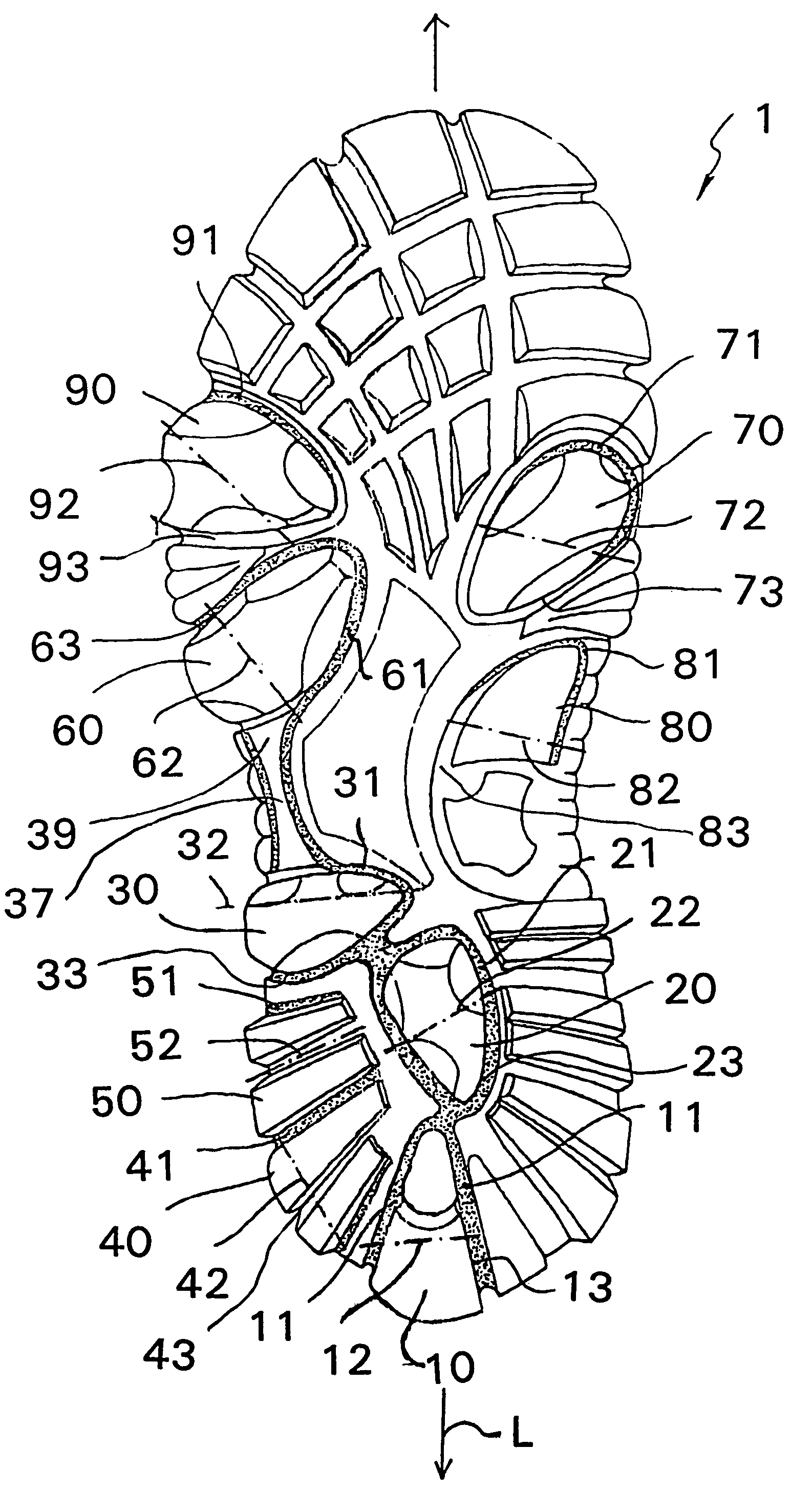
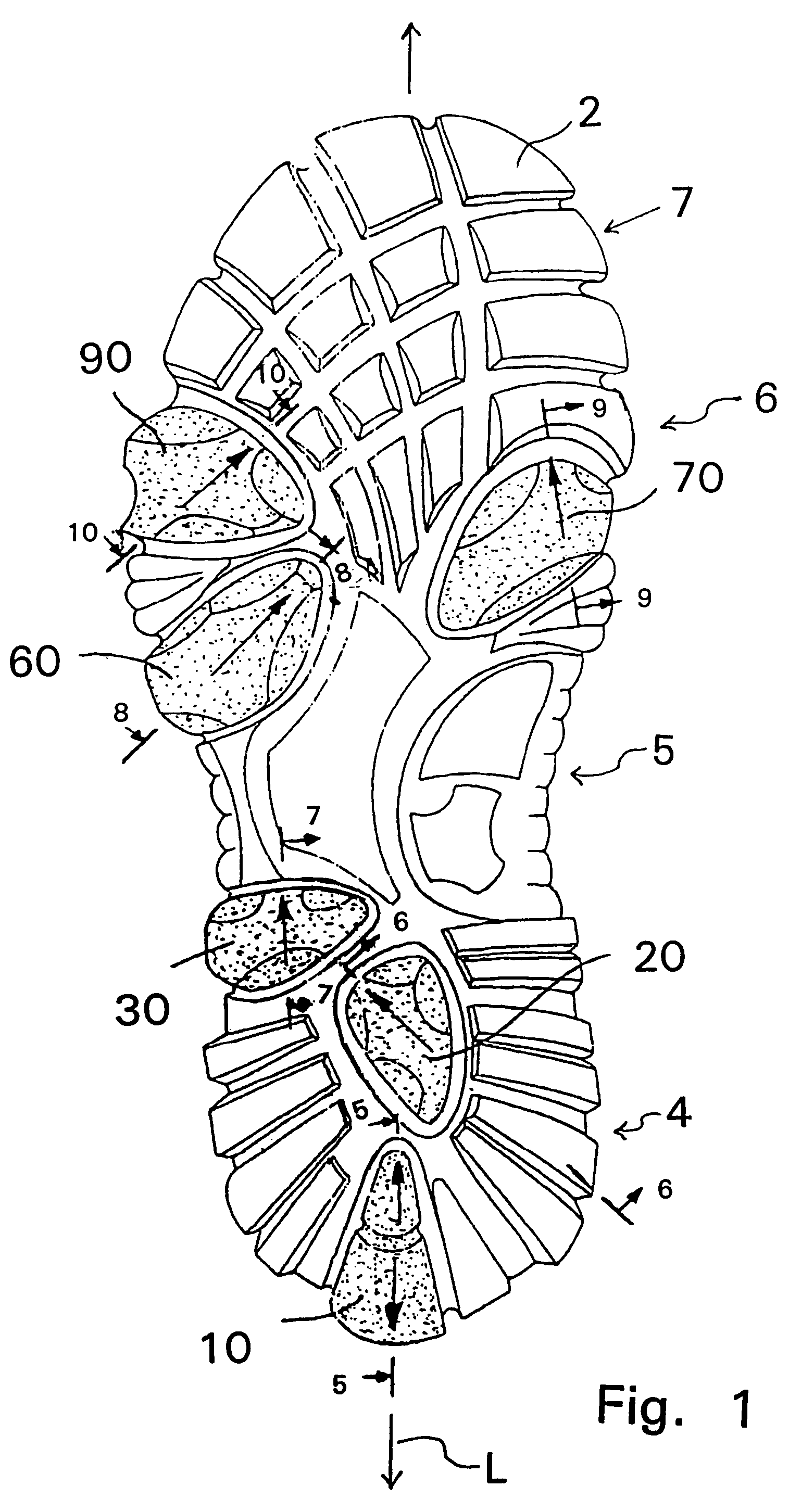
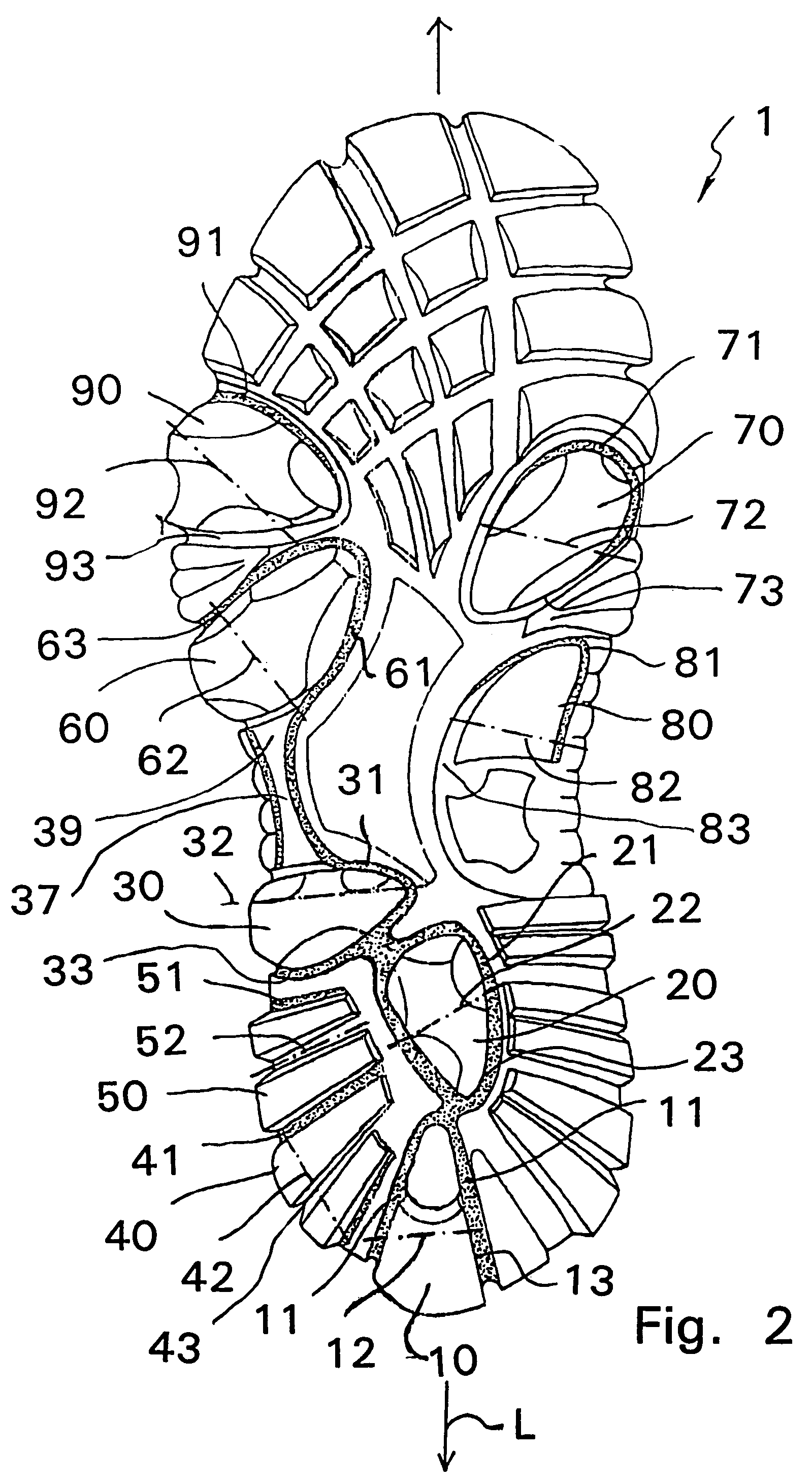
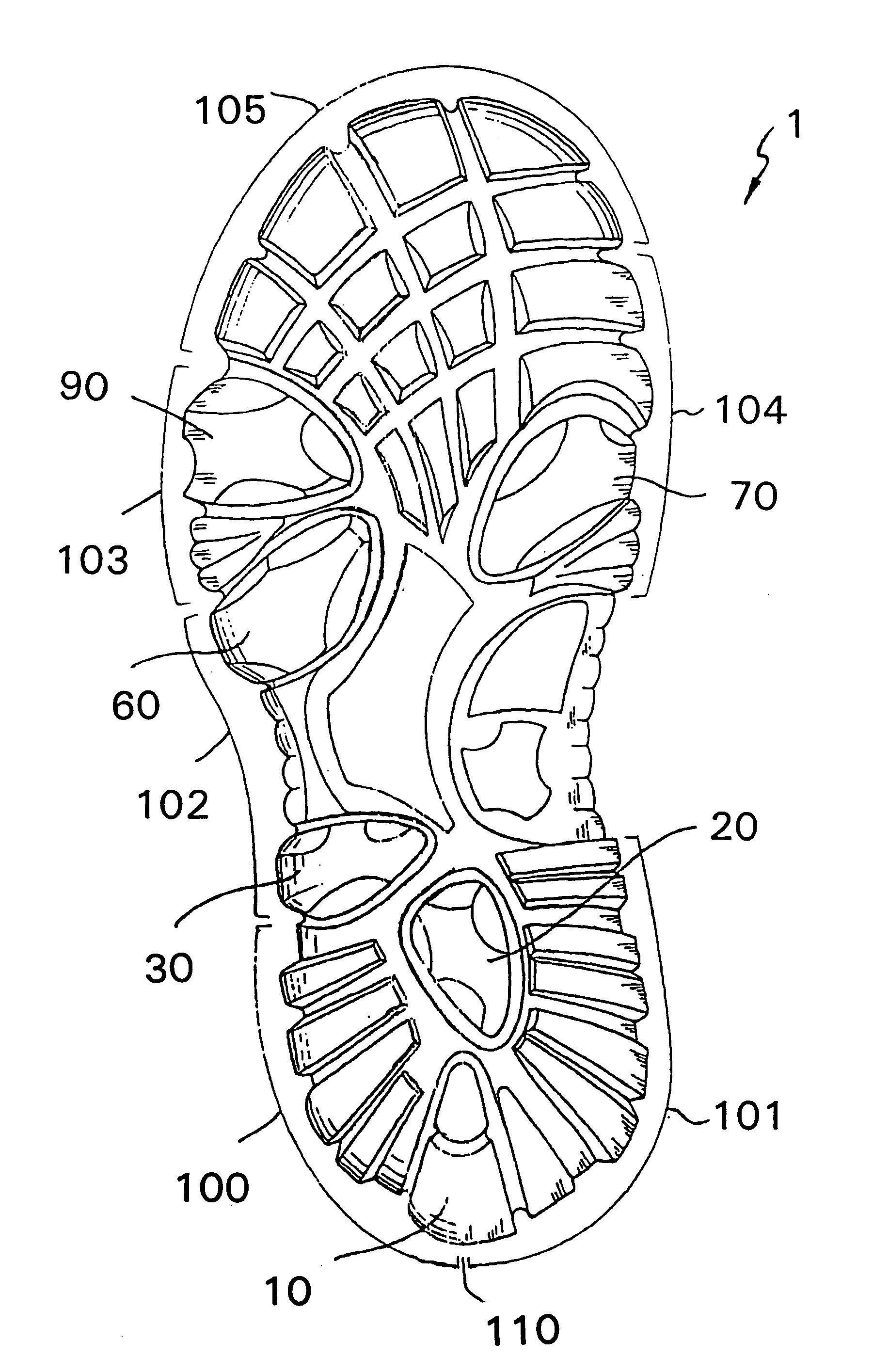
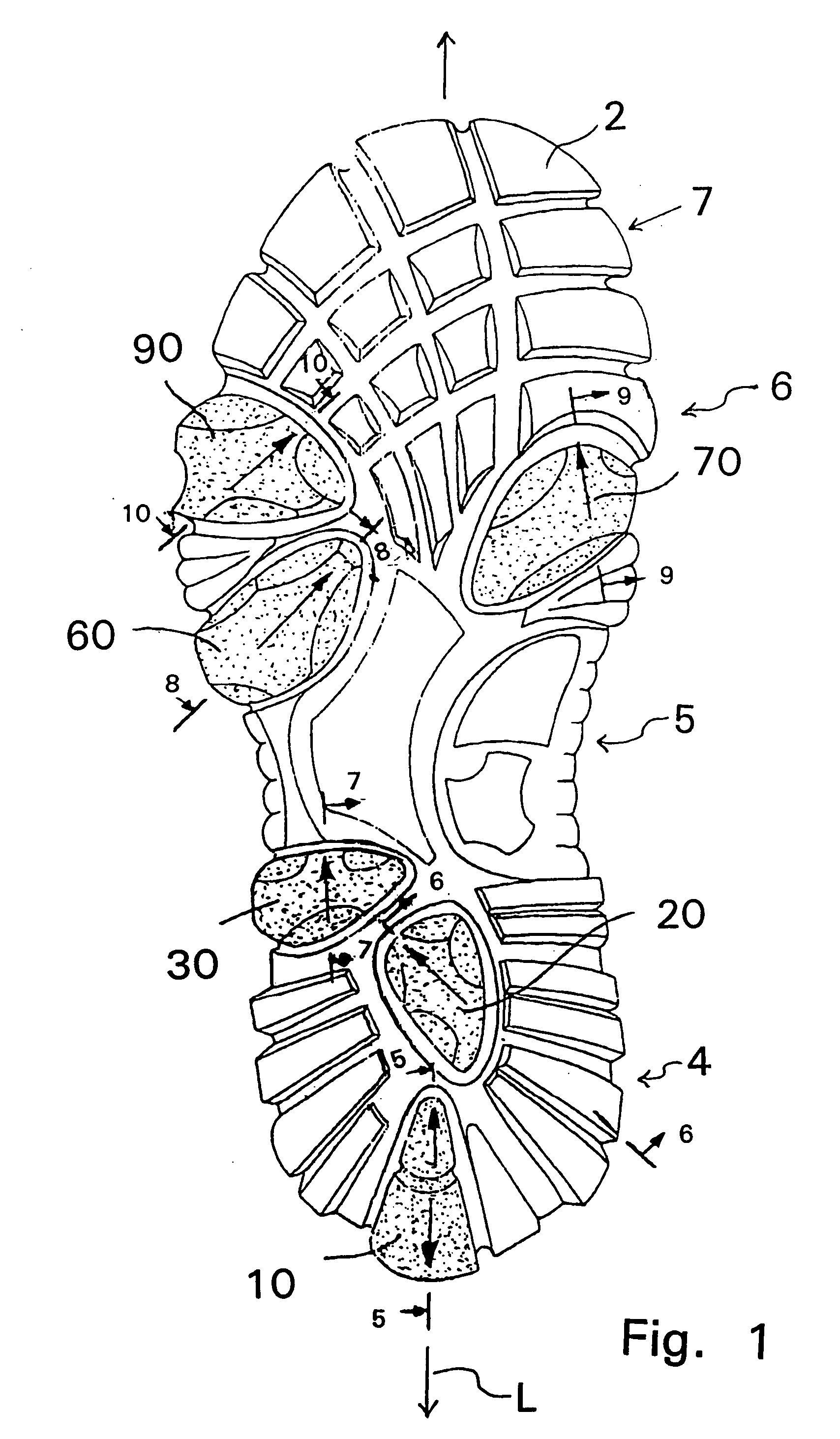
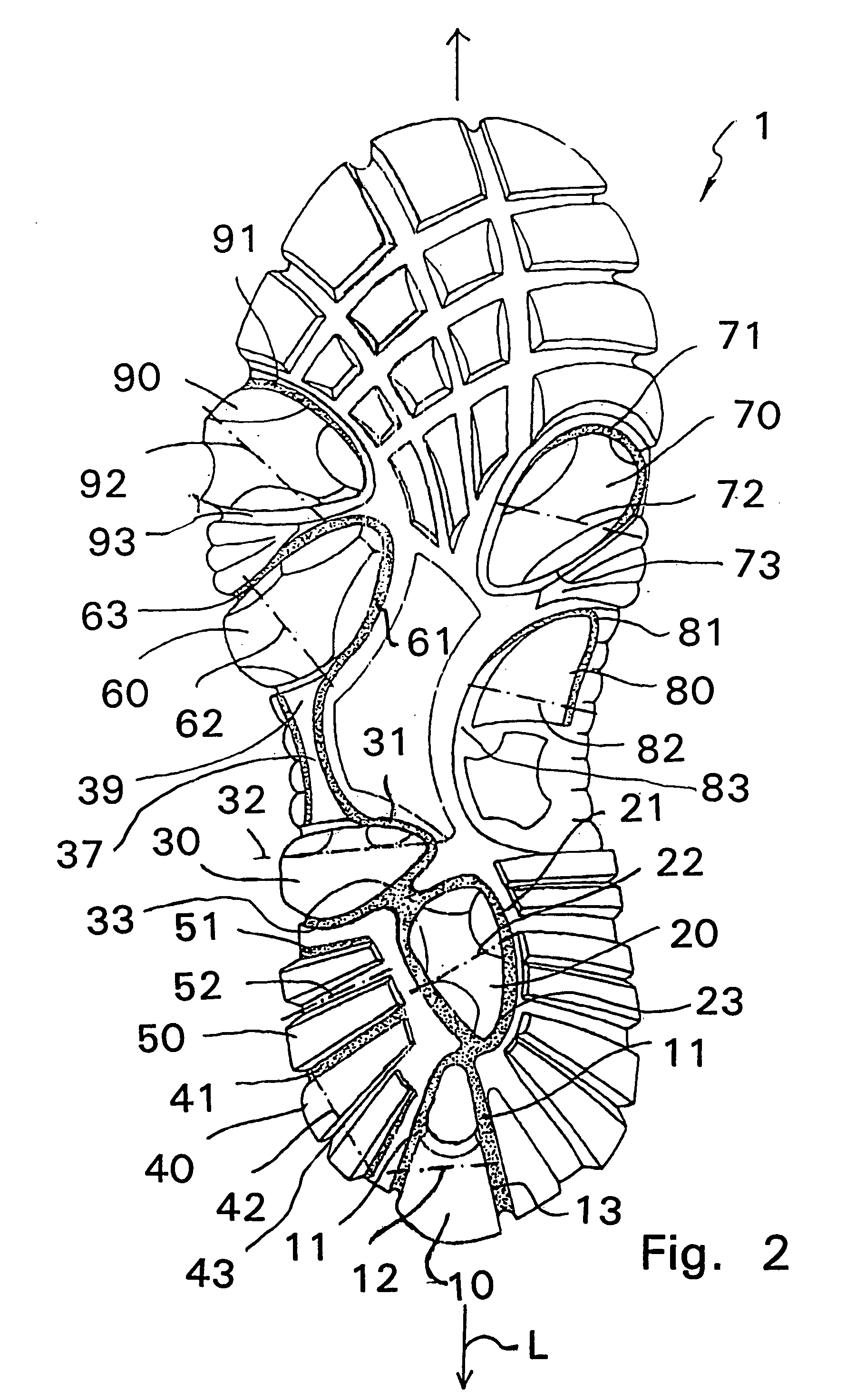
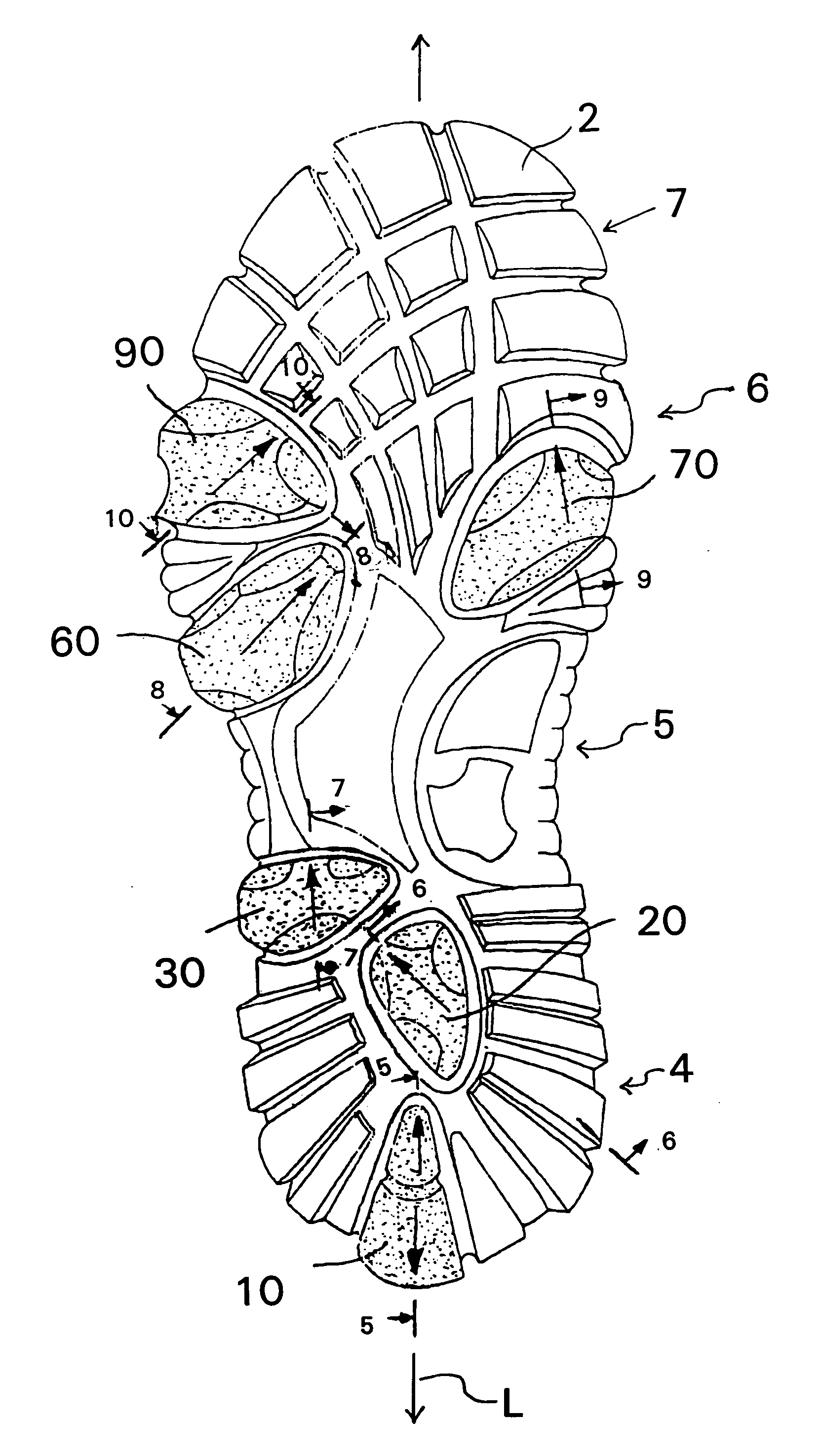
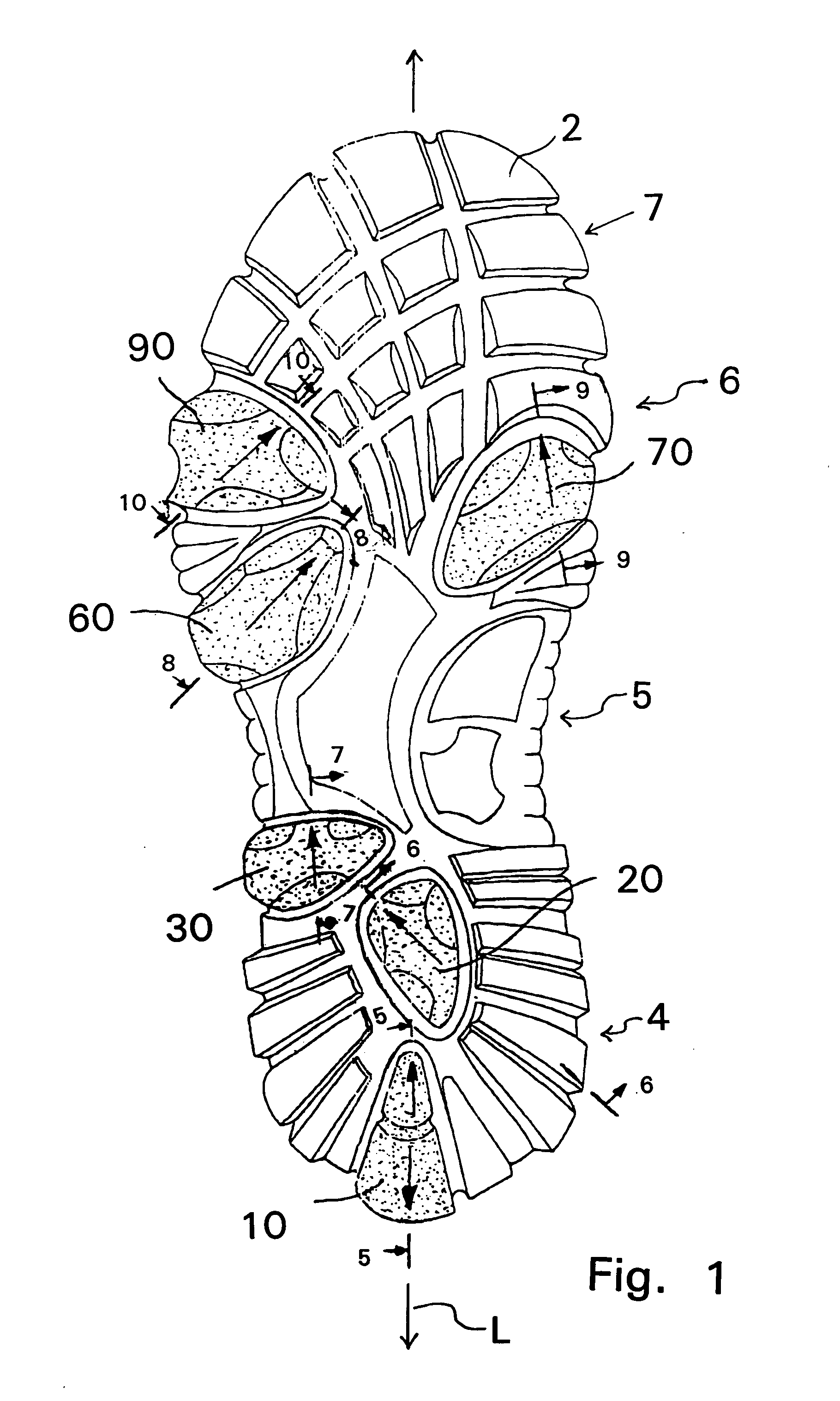
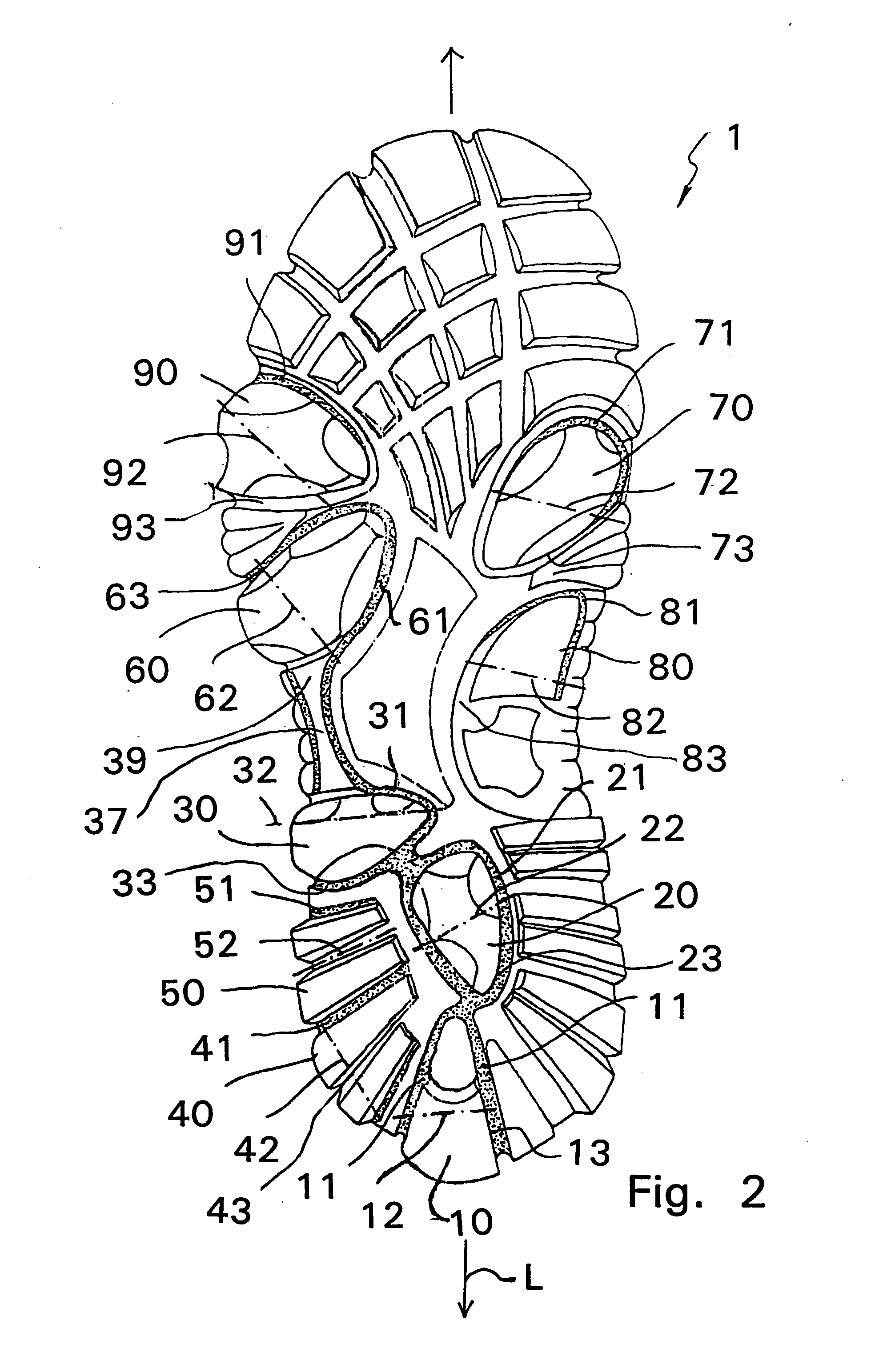
Shoe sole with foot guidance
A sole for a shoe with a foot guiding mechanism which has the particularity that the sole comprises a sole body which has, on an outer face thereof, at least one protrusion. The at least one protrusion is flexible in order to produce a desired movement of the protrusion which is suitable to force a guided sequence for a foot, wearing the shoe sole, from when the heel section initially contacts a ground surface to when the front edge of the sole breaks contact with the ground surface.
Owner:ALINE SYST INC
Shoe sole with foot guidance
A sole for a shoe with a foot guiding mechanism which has the particularity that the sole comprises a sole body which has, on an outer face thereof, at least one protrusion. The at least one protrusion is flexible in order to produce a desired movement of the protrusion which is suitable to force a guided sequence for a foot, wearing the shoe sole, from when the heel section initially contacts a ground surface to when the front edge of the sole breaks contact with the ground surface.
Owner:ALINE SYST INC
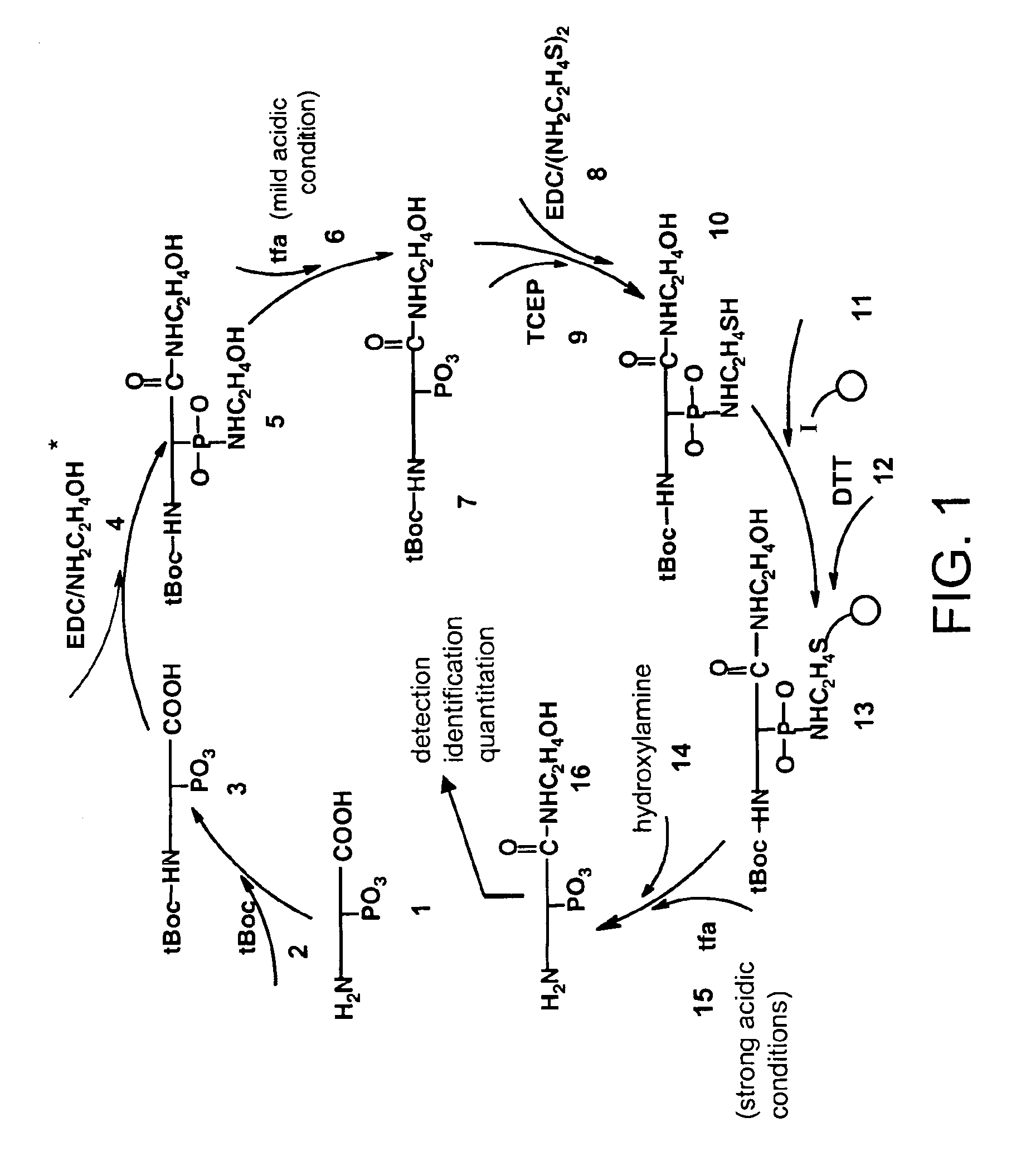
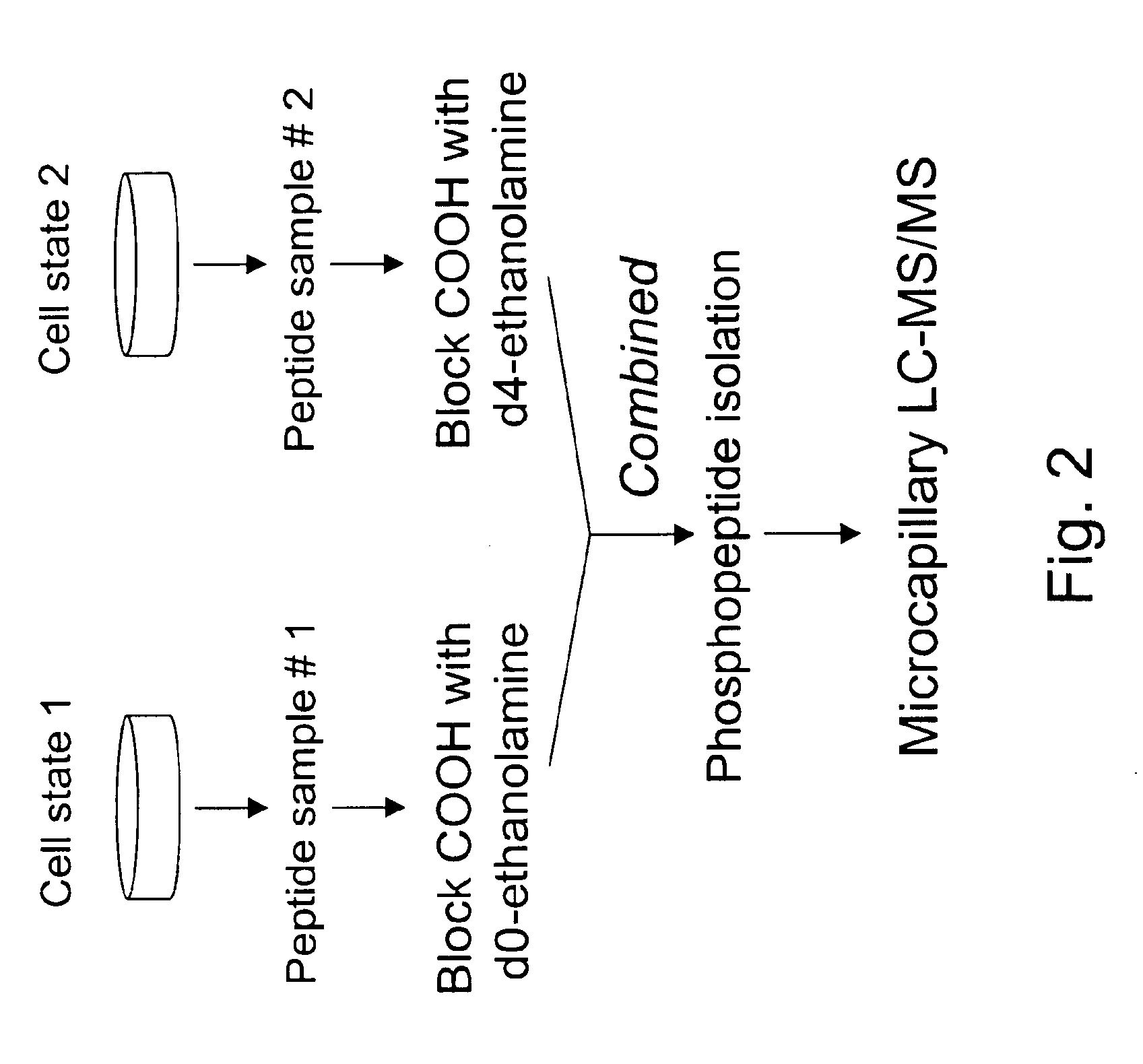
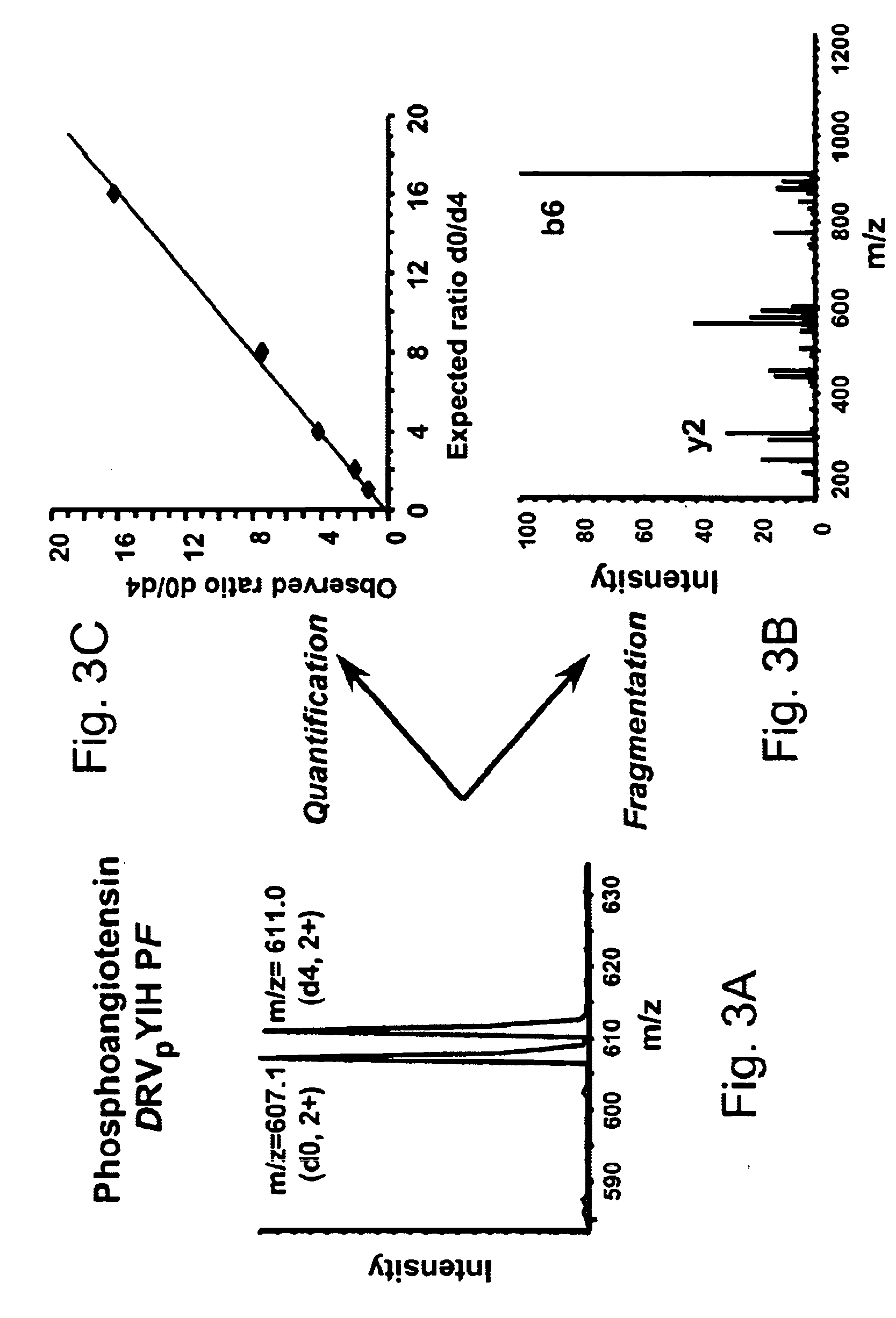
Selective labeling and isolation of phosphopeptides and applications to proteome analysis
InactiveUS7052915B2Easy to separateImprove isolationComponent separationMaterial analysis by electric/magnetic meansPhosphatePhosphoric acid
A method for selective labeling of phosphate groups in natural and synthetic oligomers and polymers in the presence of chemically related groups such as carboxylic acid groups. The method is specifically applicable to biological oligomers and polymers, including phosphopeptides, phosphoproteins and phospholipids. In a specific embodiment, selective labeling of phosphate groups in proteins and peptides, for example, facilitates separation, isolation and detection of phosphoproteins and phosphopeptides in complex mixtures of proteins. Selective labeling can be employed to selectively introduce phosphate labels at phosphate groups in an oligomer or polymer, e.g., in a peptide or protein. Detection of the presence of the label, is used to detect the presence of the phosphate group in the oligomer or polymer. The method is useful for the detection of phosphoproteins or phosphopeptides. The phosphate label can be a colorimetric label, a radiolabel, a fluorescent or phosphorescent label, an affinity label or a linker group carrying a reactive group (or latent reactive group) that allows selective attachment of the oligomer or polymer (protein or peptide) to a phosphate label, to an affinity label or to a solid support. The method can be combined with well-known methods of mass spectrometry to detect and identify phosphopeptides and phosphoproteins.
Owner:UNIV OF WASHINGTON
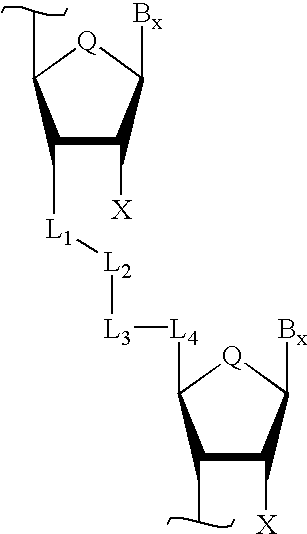
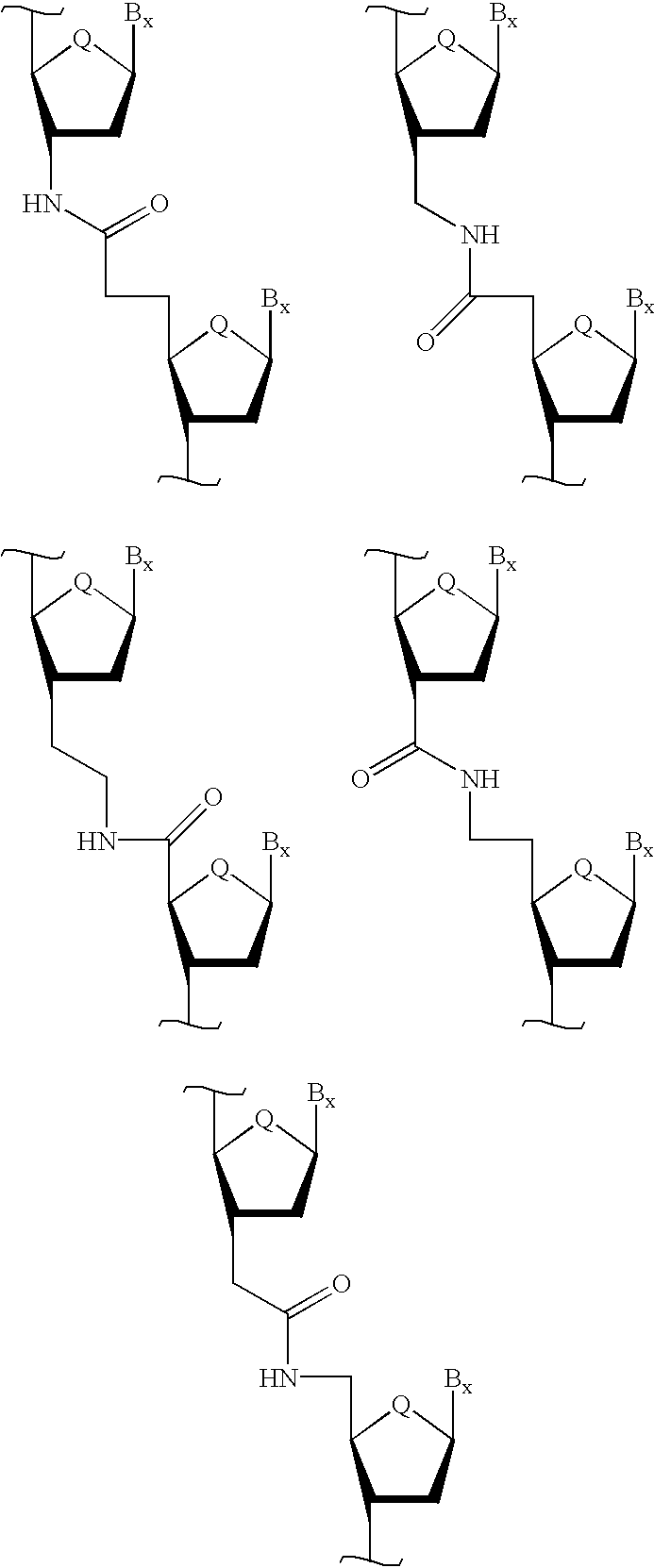
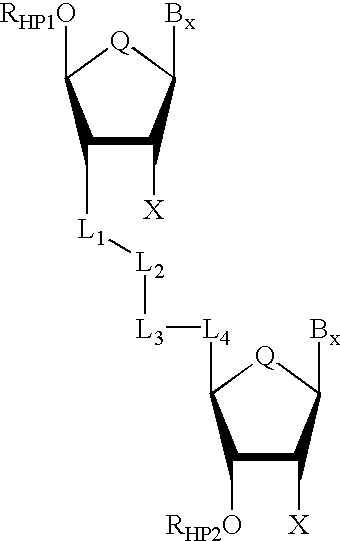
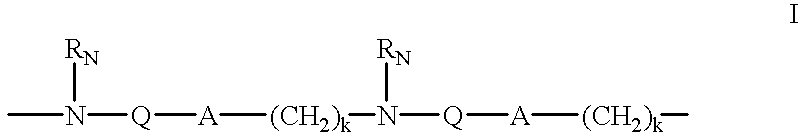
Backbone modified oligonucleotide analogues
InactiveUS20030045705A1Improved pharmacokinetic propertiesImprove propertiesEsterified saccharide compoundsCompounds screening/testingOligomerRna expression
Therapeutic oligonucleotide analogues which have improved nuclease resistance and improved cellular uptake are provided. Replacement of the normal phosphorodiester inter-sugar linkages found in natural oligomers with four atom linking groups forms unique di- and poly-nucleosides and nucleotides useful in regulating RNA expression and in therapeutics. Methods of synthesis and use are also disclosed.
Owner:IONIS PHARMA INC
Backbone modified oligonucleotide analogues
InactiveUS6900301B2Improve stabilitySequencing is facilitatedCompounds screening/testingEsterified saccharide compoundsOligomerRna expression
Therapeutic oligonucleotide analogues which have improved nuclease resistance and improved cellular uptake are provided. Replacement of the normal phosphorodiester inter-sugar linkages found in natural oligomers with four atom linking groups forms unique di- and poly-nucleosides and nucleotides useful in regulating RNA expression and in therapeutics. Methods of synthesis and use are also disclosed.
Owner:IONIS PHARMA INC
Oligonucleoside linkages containing adjacent nitrogen atoms
InactiveUS6214551B1Sequencing is facilitatedConfer nuclease resistanceGroup 4/14 element organic compoundsSugar derivativesNitrogenOligonucleotide
Novel compounds that mimic and / or modulate the activity of wild-type nucleic acids. In general, the compounds contain a selected nucleoside sequence wherein the nucleosides are covalently bound through linking groups that contain adjacent nitrogen atoms.
Owner:IONIS PHARMA INC
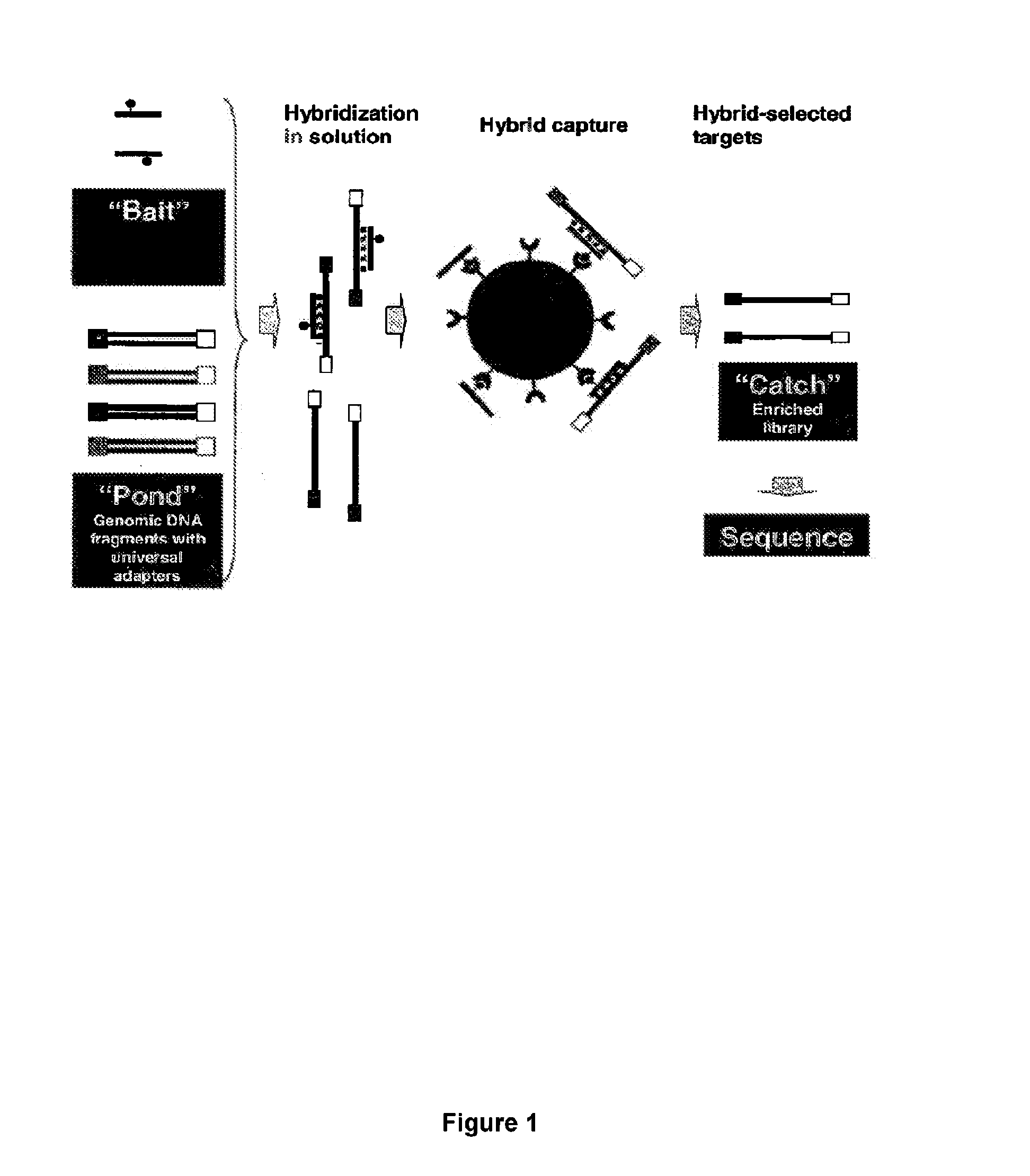
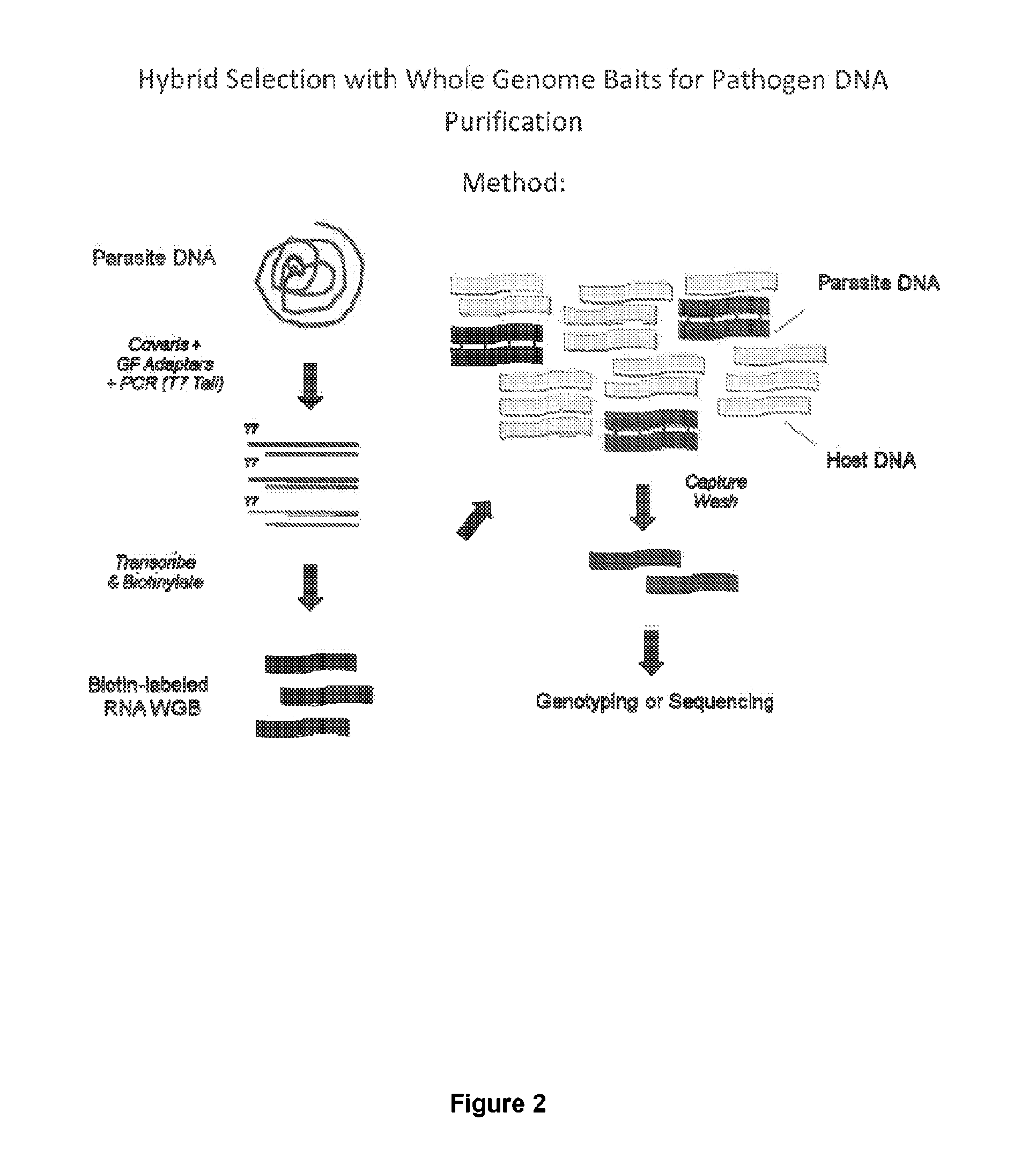
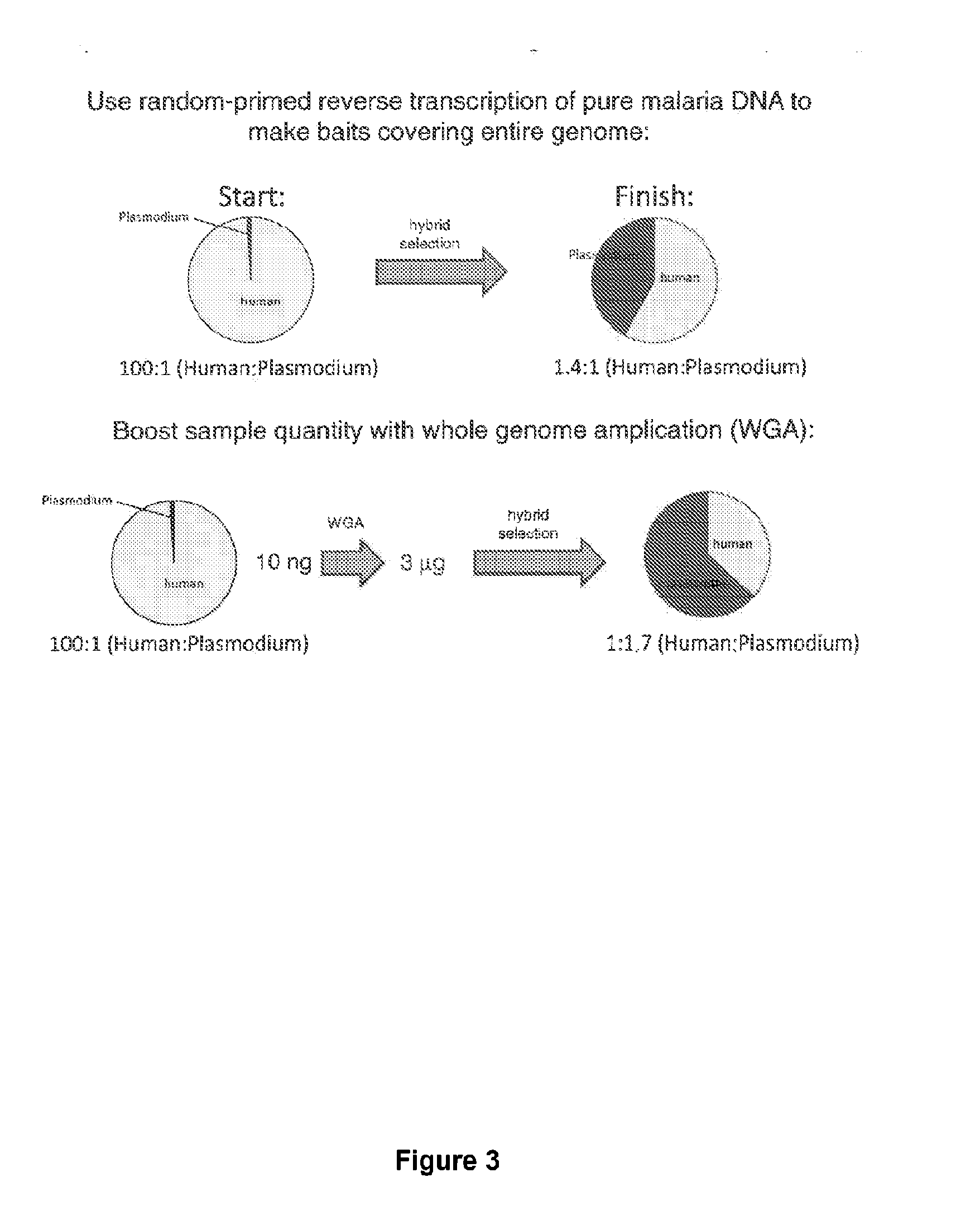
Hybrid selection using genome-wide baits for selective genome enrichment in mixed samples
InactiveUS20130230857A1Cost-effectiveAugment sequencing coverage depthSugar derivativesMicrobiological testing/measurementGenotypingMalaria
The present invention provides methods for sequencing and genotyping of DNA useful for analysis of samples in which the target DNA represents a small portion (e.g., 10-1000-fold less) that a contaminating DNA source. Accordingly, the methods described herein are useful for sequencing or genotyping pathogen DNA, such as malaria DNA, in clinical samples taken from infected subjects.
Owner:THE BROAD INST INC
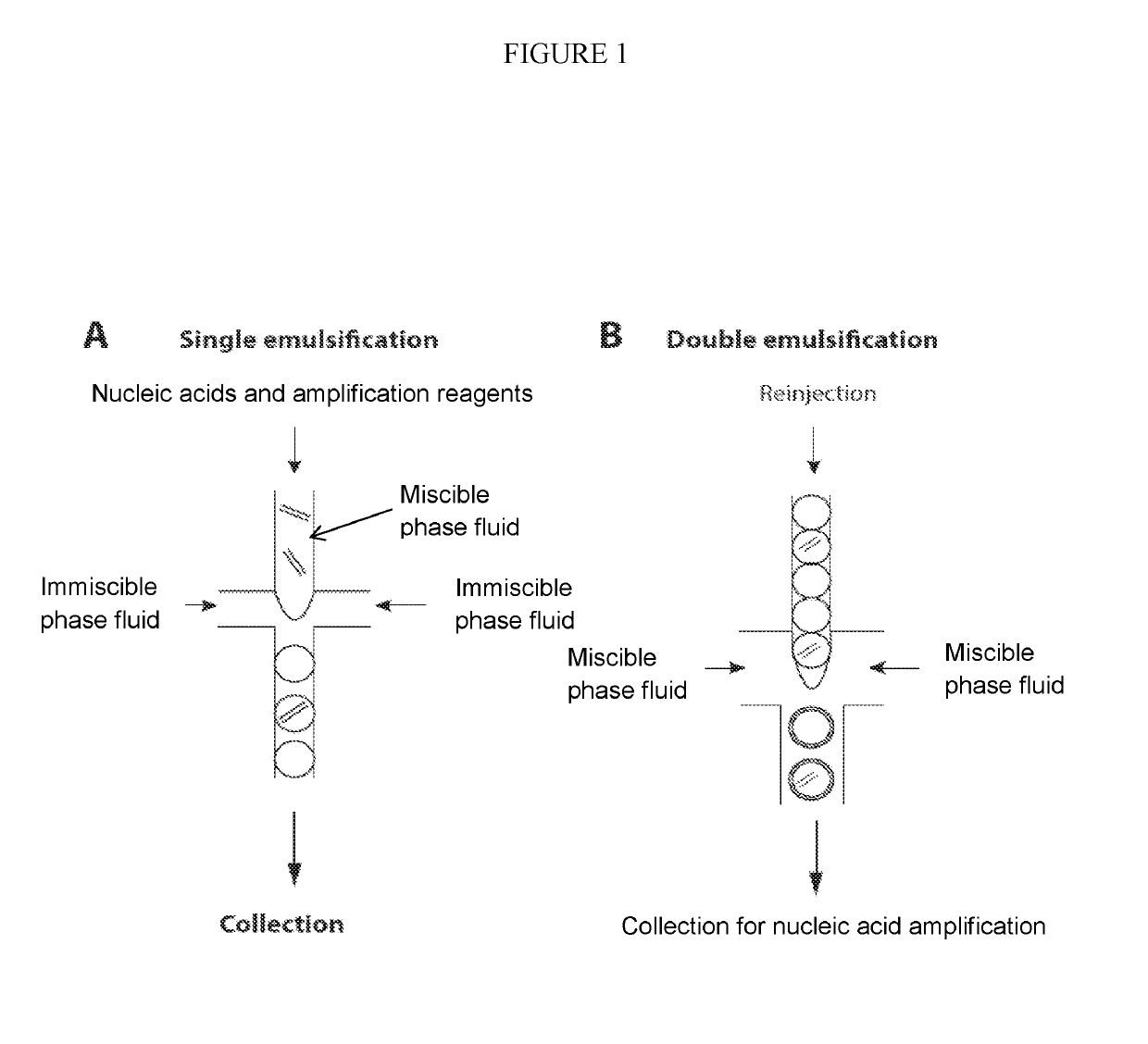
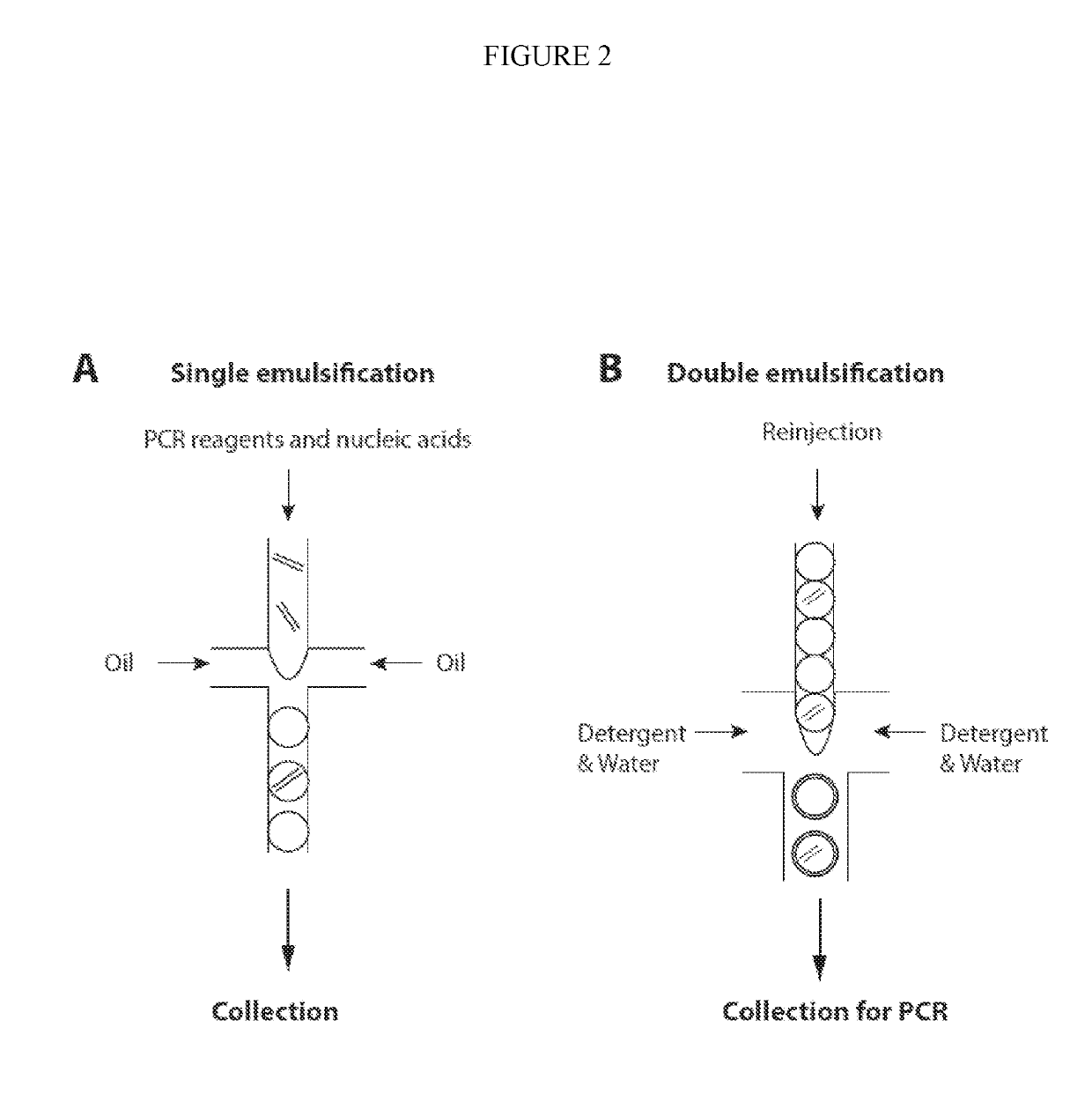
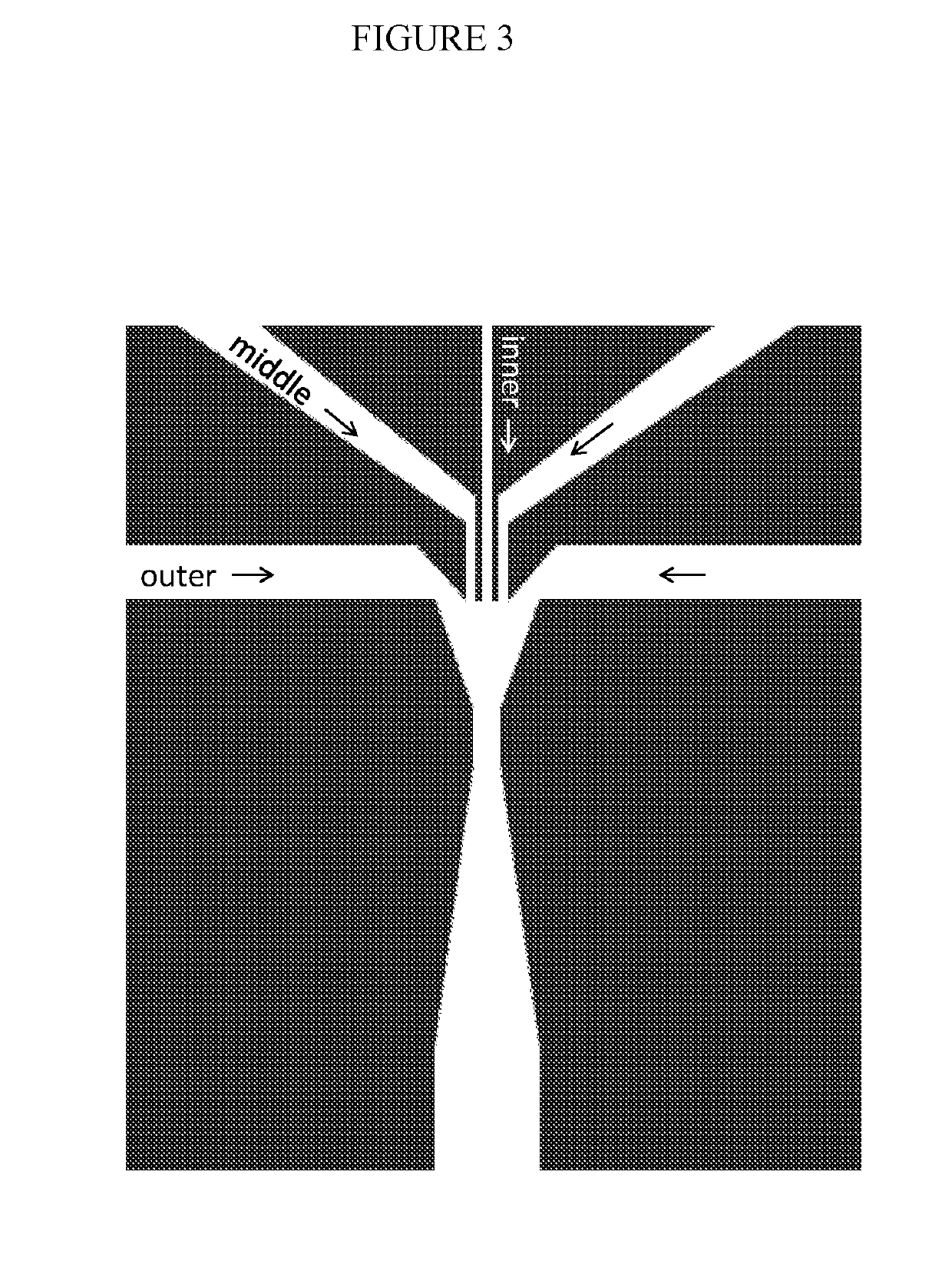
Combined multiple-displacement amplification and PCR in an emulsion microdroplet
ActiveUS20190218594A1Easy to identifySequencing is facilitatedHeating or cooling apparatusMicrobiological testing/measurementNucleic acid amplification techniqueVirus
The methods and systems described herein provide an improved emulsion droplet based nucleic acid amplification method, which allows nucleic acids contained in biological systems to be detected, quantitated and / or sorted based on their sequence as detected with nucleic acid amplification techniques, e.g., polymerase chain reaction (PCR). The nucleic acids can be free floating or contained within living or nonliving structures, including particles, viruses, and cells. The nucleic acids can include, e.g., DNA or RNA.
Owner:RGT UNIV OF CALIFORNIA
Genes and polymorphisms associated with AMD
InactiveUS20100303832A1Decreased and increased riskIncreased riskOrganic active ingredientsSenses disorderCvd riskFhit gene
The invention relates to genes, gene polymorphisms, and genetic profiles associated with an elevated or a reduced risk of alternative complement cascade deregulation disease such as AMD. The invention provides methods and reagents for determination of risk, diagnosis and treatment of such diseases. In an embodiment, the present invention provides methods and reagents for determining sequence variants in the genome of a patient which facilitate assessment of risk for developing such diseases.
Owner:UNIV OF IOWA RES FOUND
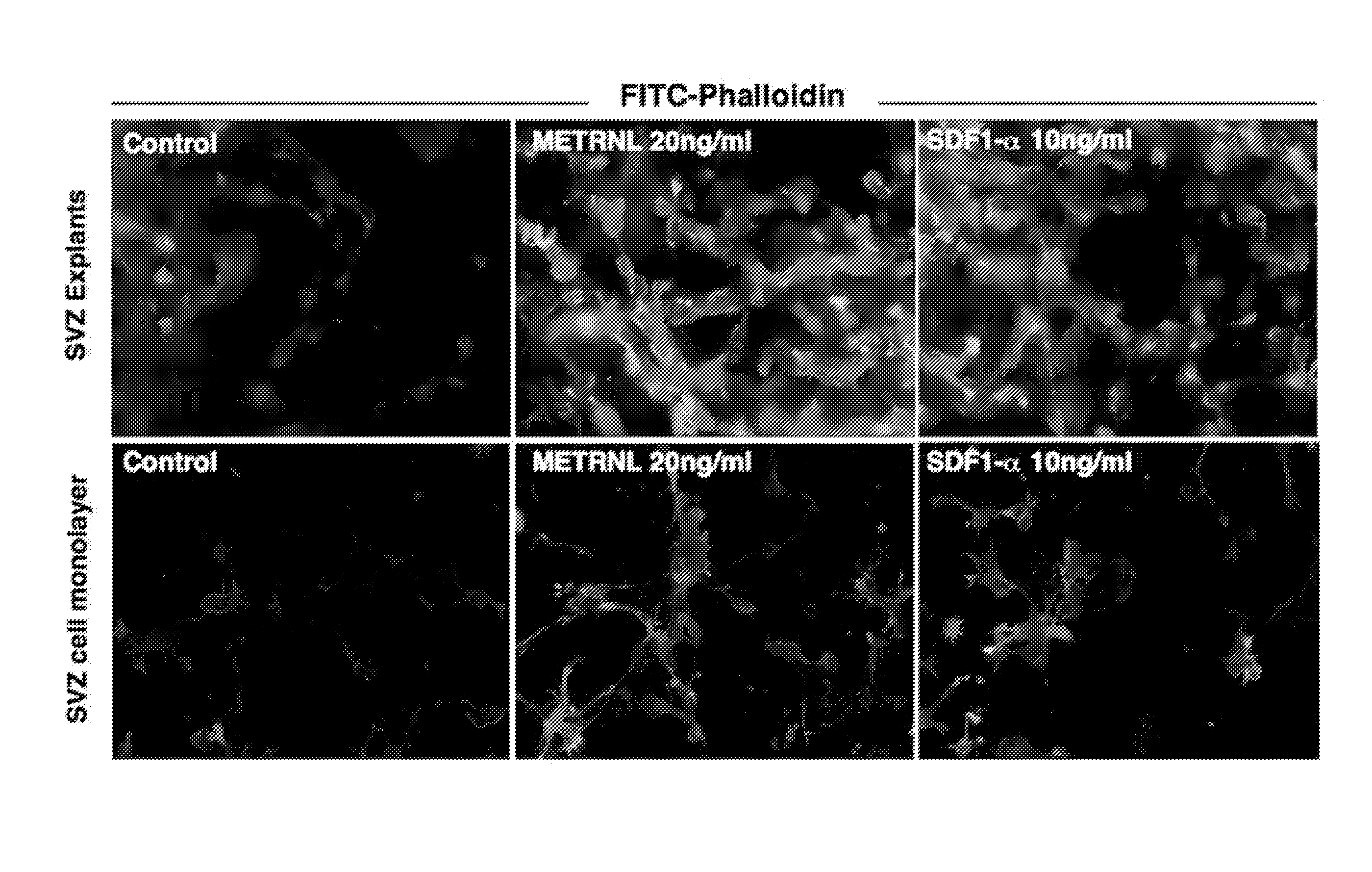
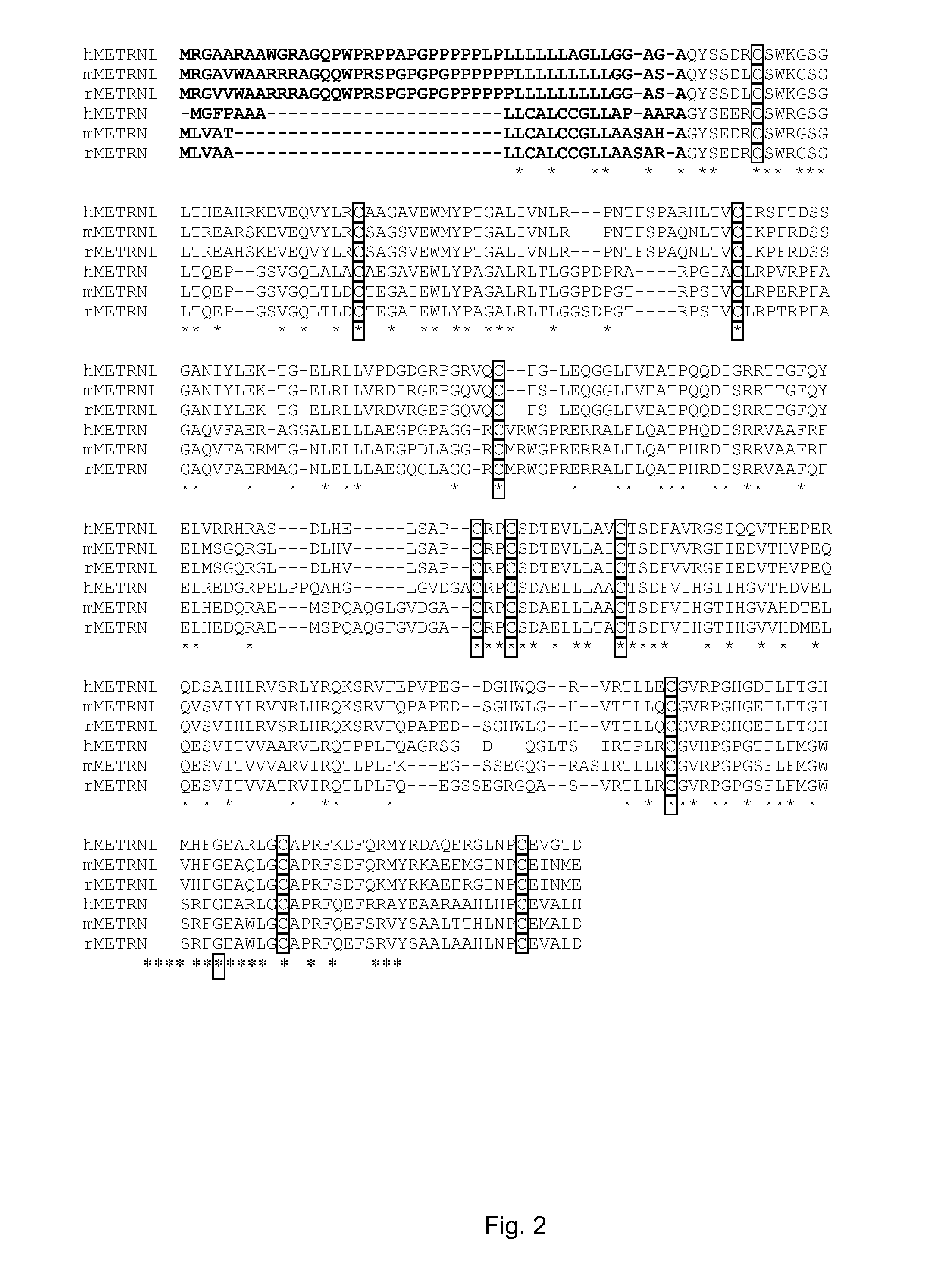
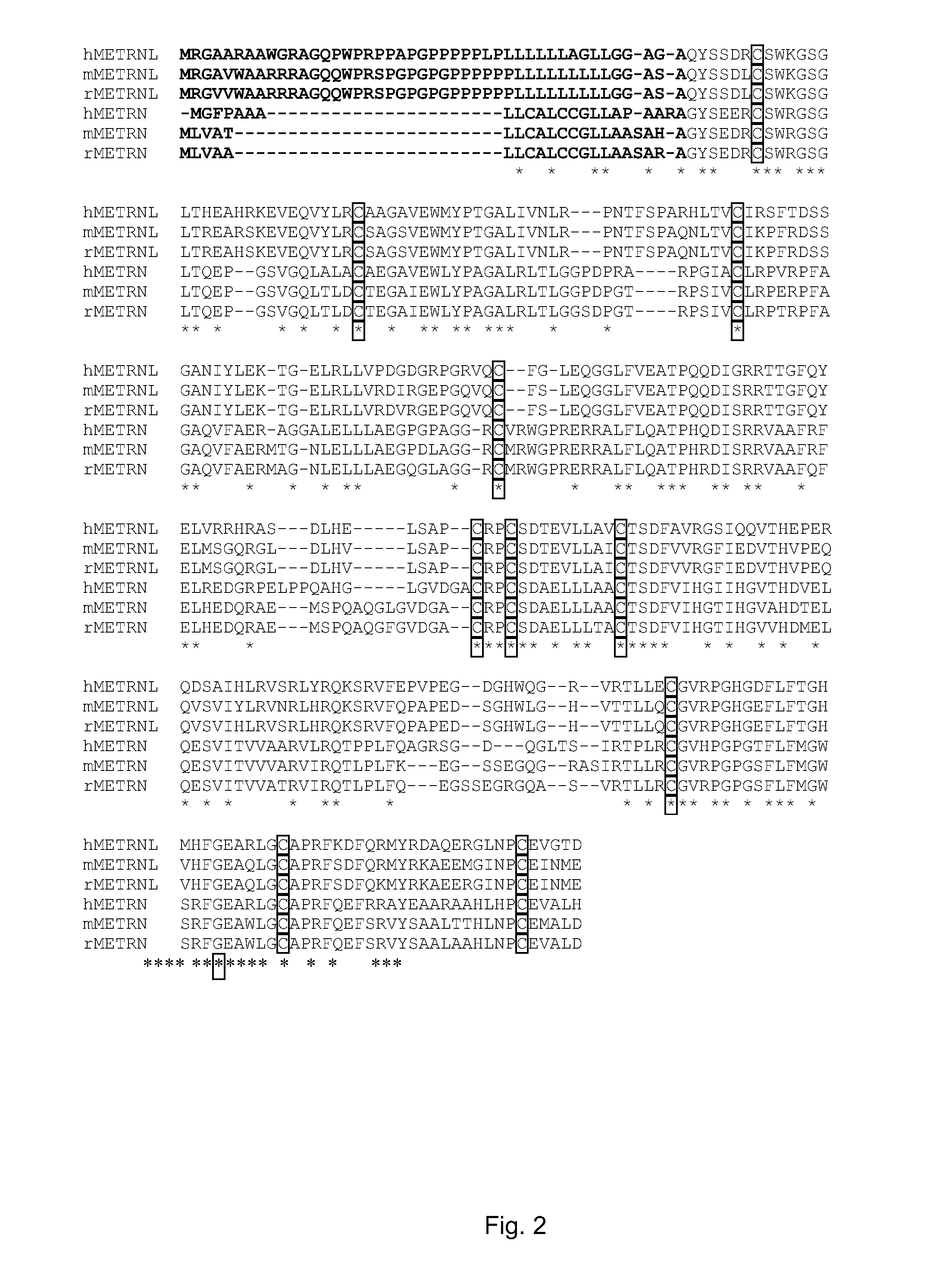
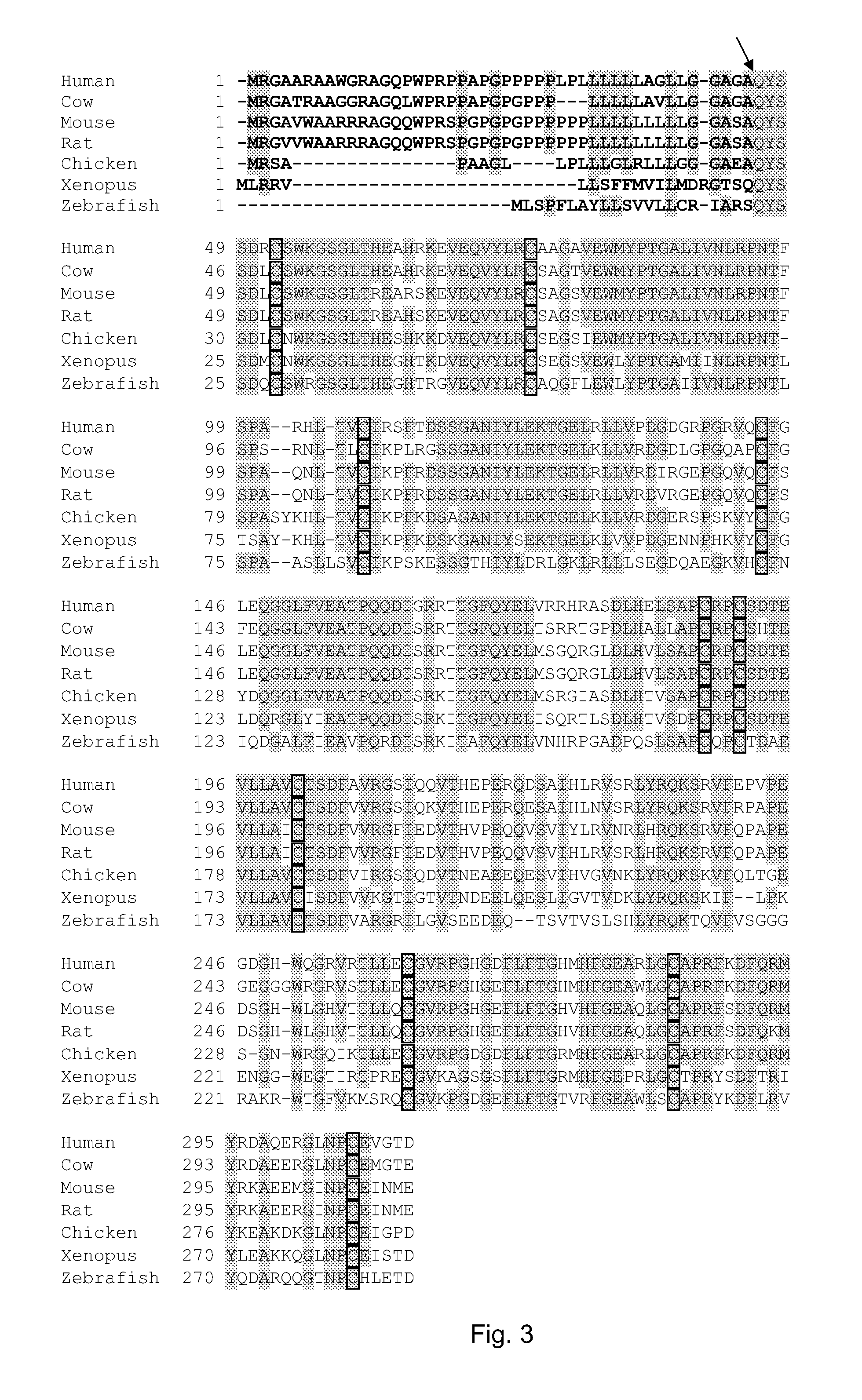
Therapeutic use of a growth factor, metrnl
ActiveUS20110112035A1Promoting axonal extensionPromote migrationNervous disorderPeptide/protein ingredientsNervous systemTherapeutic protein
The present invention relates to the field of therapeutic use of proteins, genes and cells, in particular to the therapy based on the biological function of a secreted therapeutic protein, METRNL, in particular for the treatment of disorders of the nervous system. METRNL is a Nerve Survival and Growth factor with neuroprotective and / or neurogenesis effects.
Owner:HOBA THERAPEUTICS APS
Card game that orchestrates clean up and teaches organization and task-related skills
A method of playing an interactive card game for orchestrating clean up of a space and teaching organization and task-related skills. The card game includes a deck of cards composed of cards describing and depicting respective activities to be executed by at least one player and cards depicting rewards; and objects which facilitate playing the card game and which are at least one of present in the space and supplied with the card game. The card game is used for orchestrating clean up of the space and teaching organization including sorting and task-related skills by playing the card game which is presented in a fun and educational format so that players perceive the process of sorting and cleaning up as a pleasant experience.
Owner:LEWIS JEAN +2
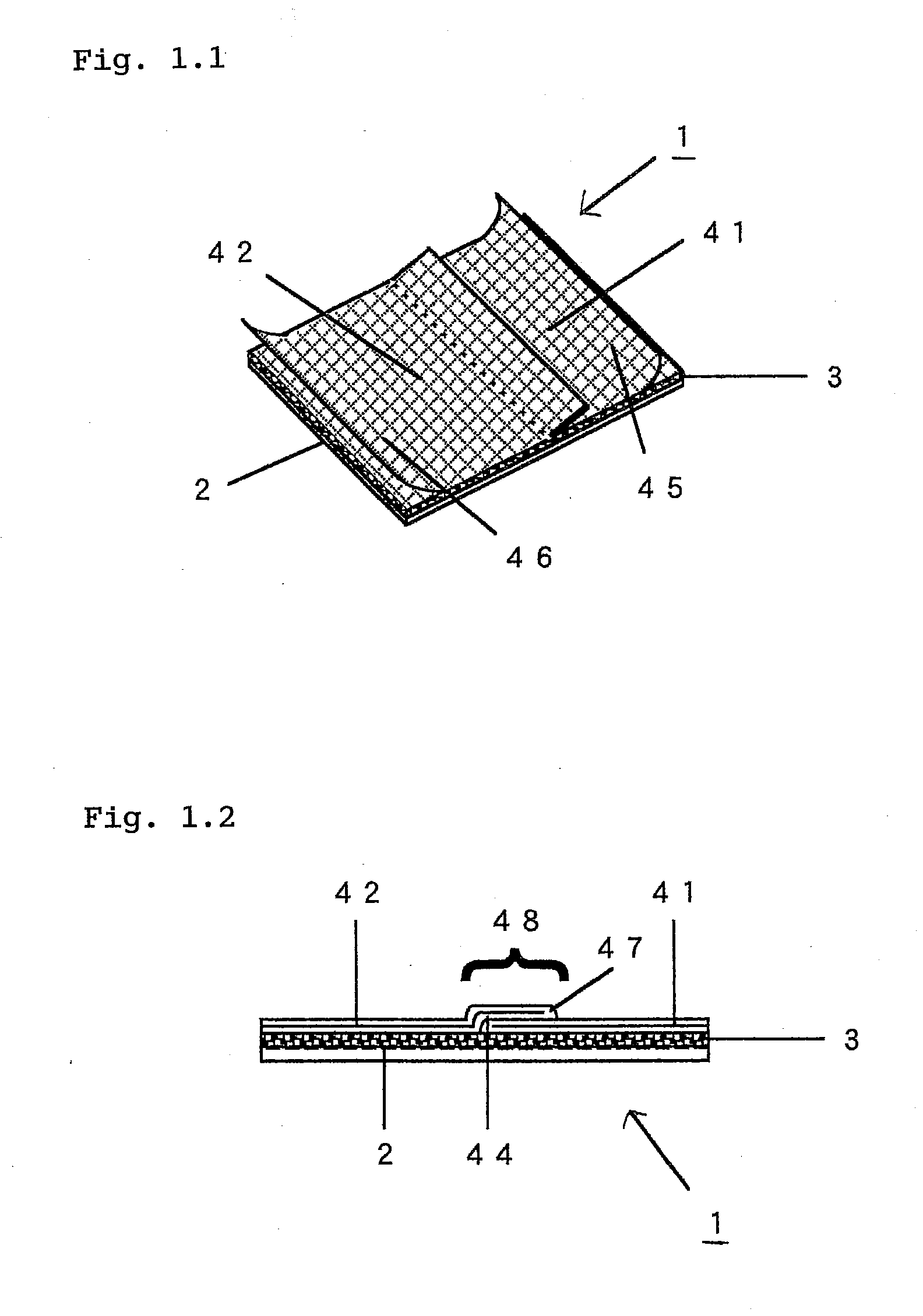
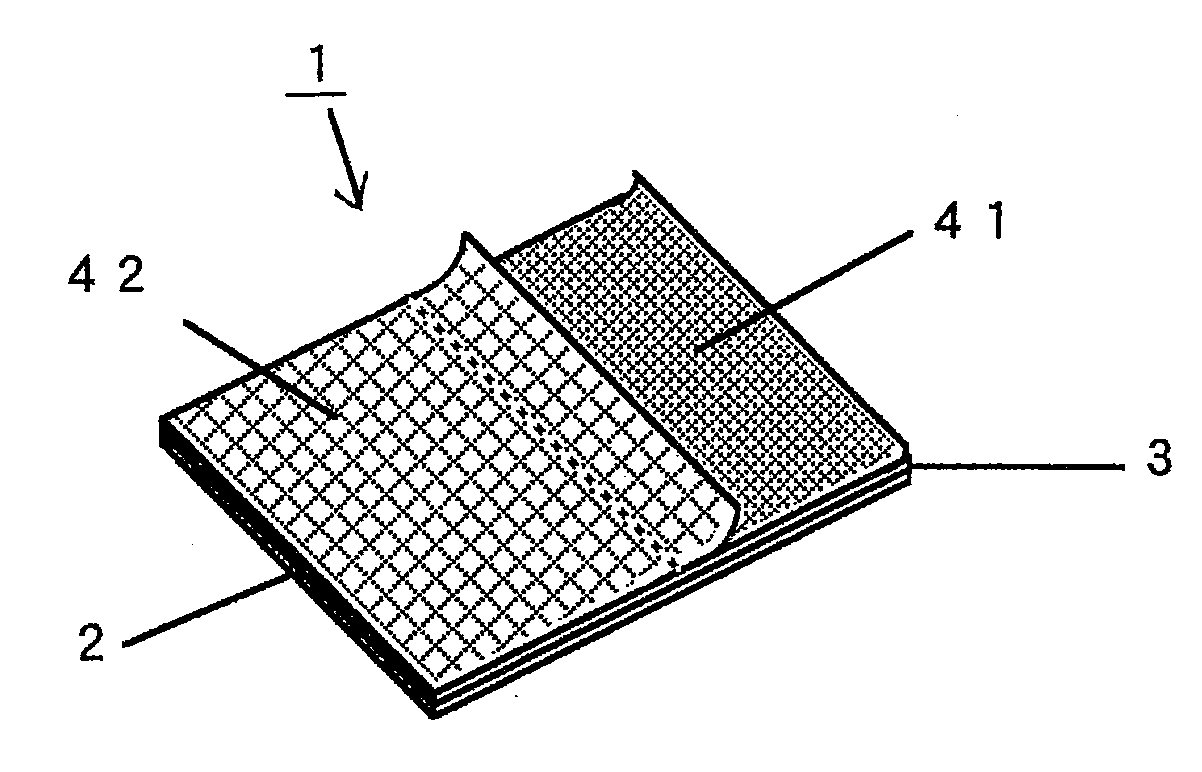
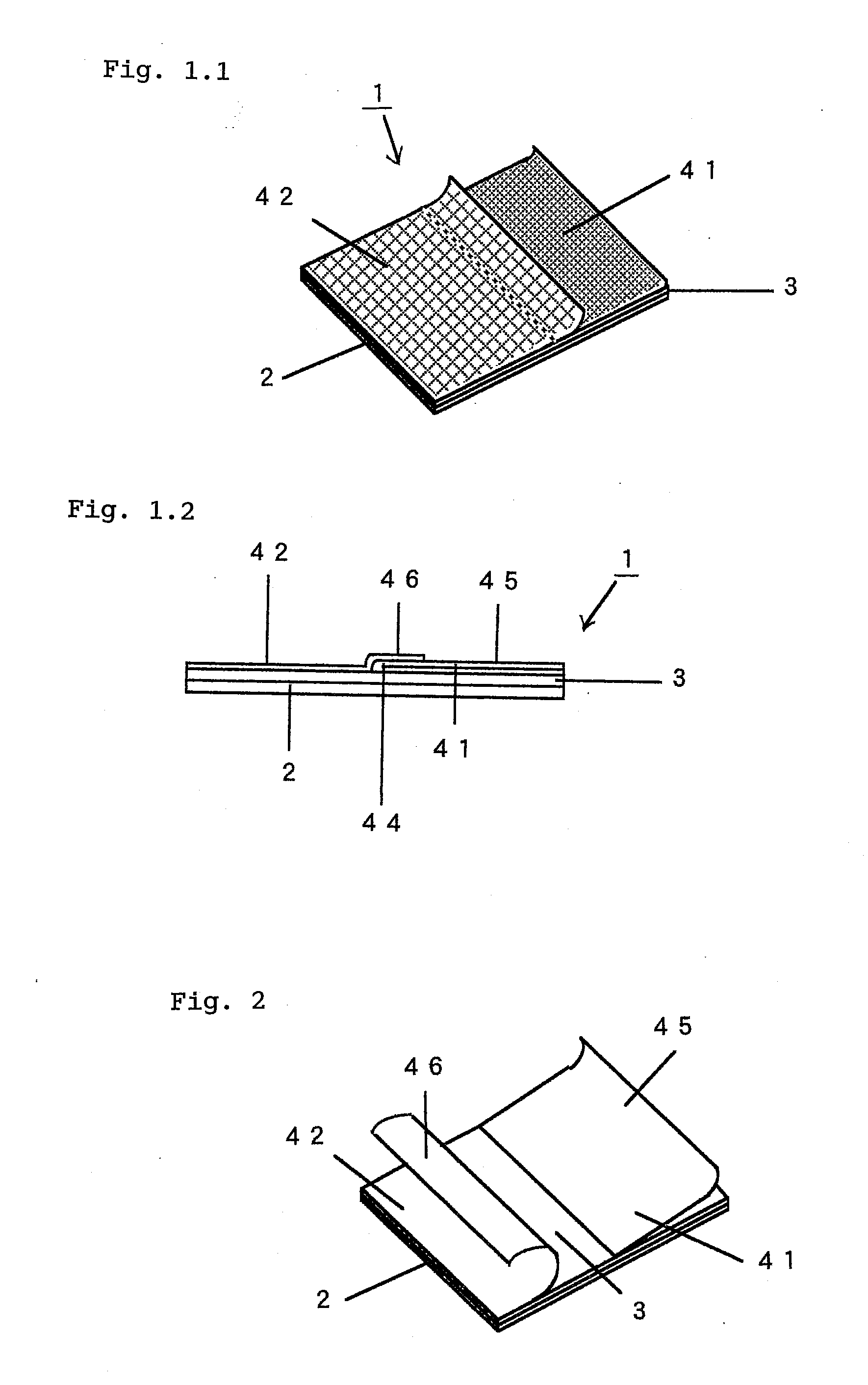
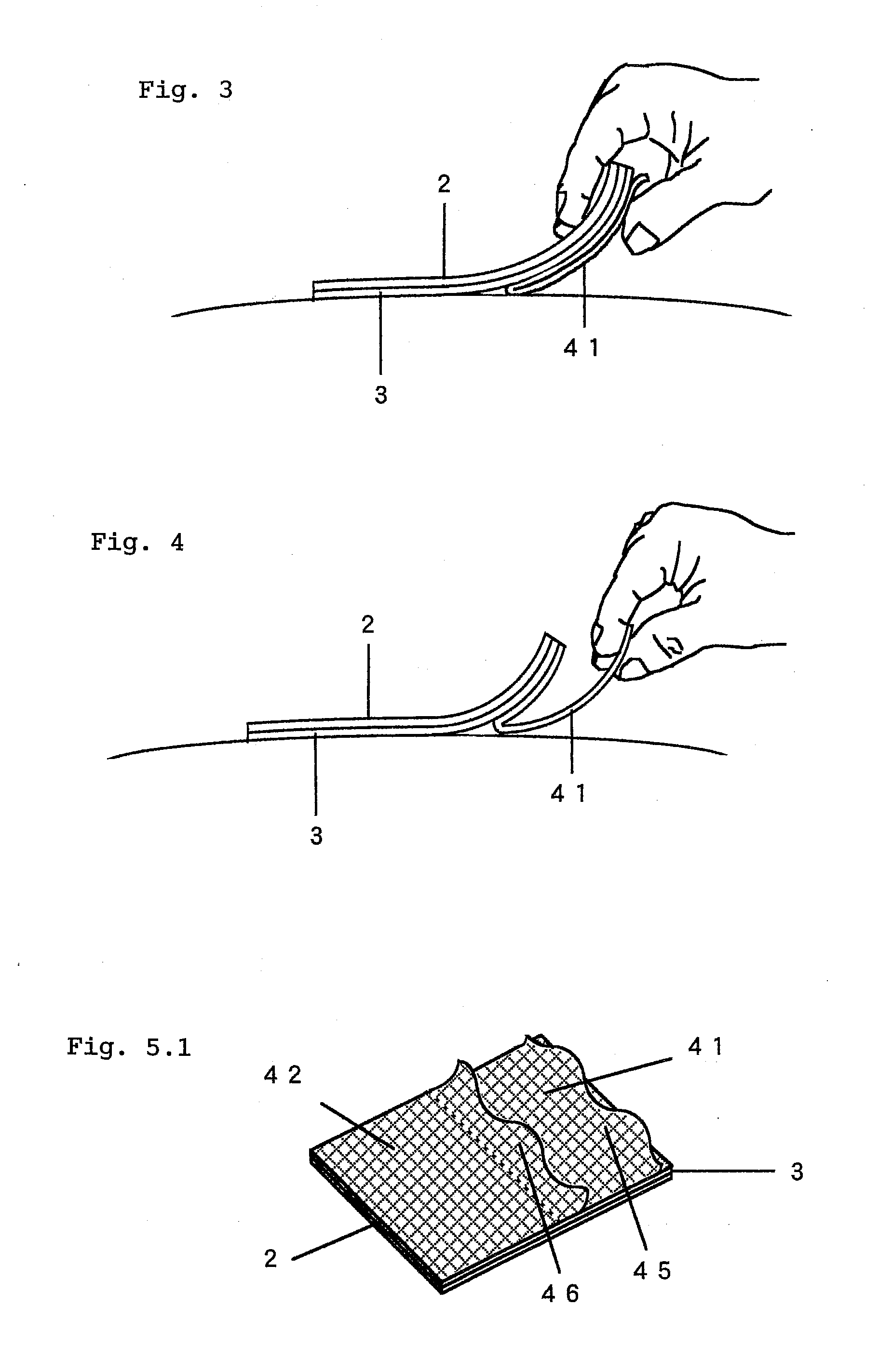
Adhesive Patch and Production Method Thereof
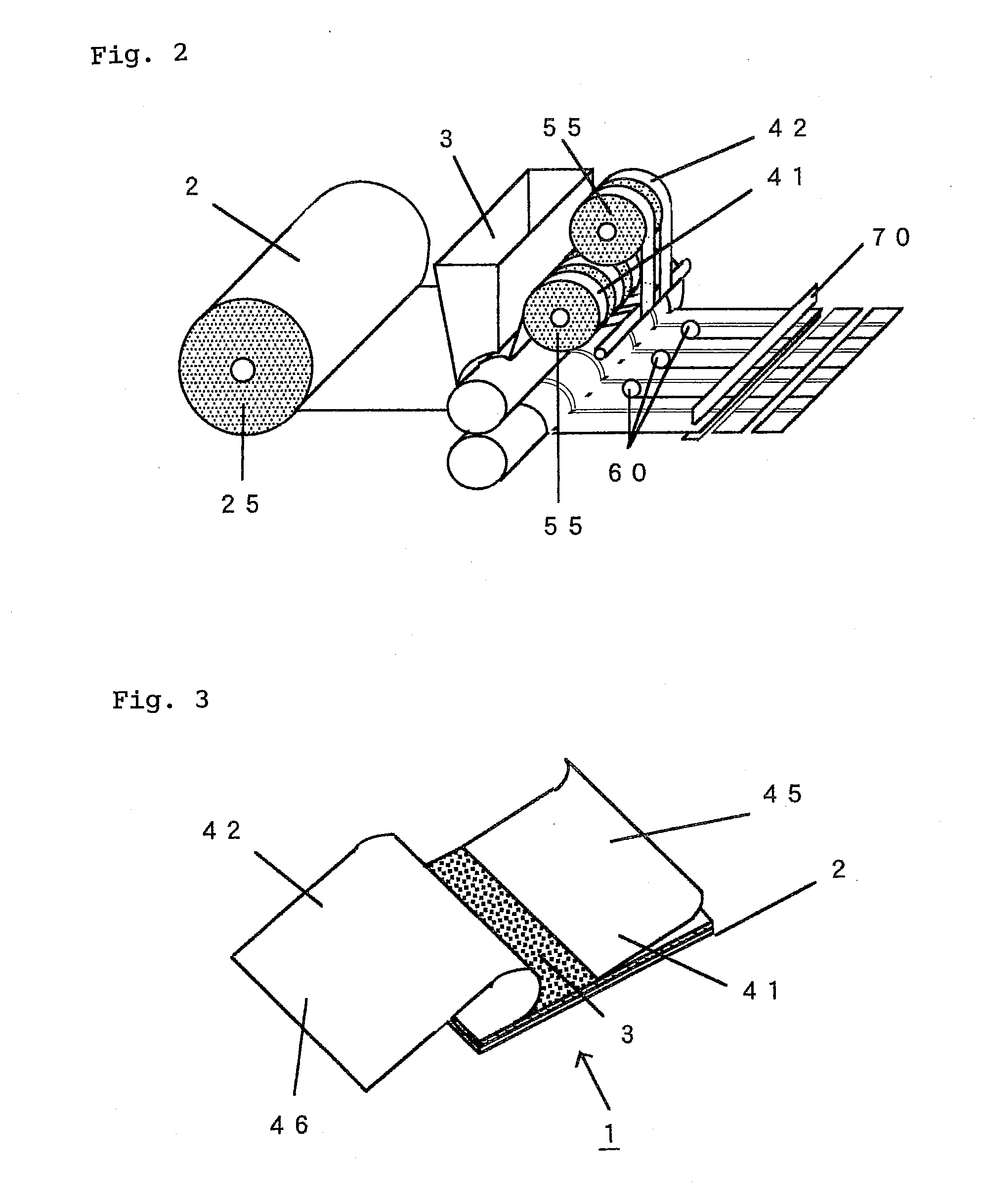
InactiveUS20100298788A1Easy to pinchSequencing is facilitatedLamination ancillary operationsPlastersMethods of productionChemistry
An adhesive patch, such as poultice-type patch, is provided, which is suitable for mass production and allows the user to apply it without touching the drug-containing matrix. A production method of such a adhesive patch is also provided.Specifically, the method for producing adhesive patch, wherein(a) the adhesive patch comprises two liners;(b) the first liner is folded at the middle thereof so that it is divided by the fold into two sections that together form a V-shaped liner, one of the two sections adhering to the drug-containing matrix surface; and(c) the second liner adheres to the remaining part of the drug-containing matrix surface with one end of the second liner covering the fold of the V-shaped first liner, the method including:allowing a tubular liner to adhere to at least half of the surface of the drug-containing matrix spread over the backing; andcutting the tubular liner in half to form the V-shaped first liner folded at the middle thereof.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
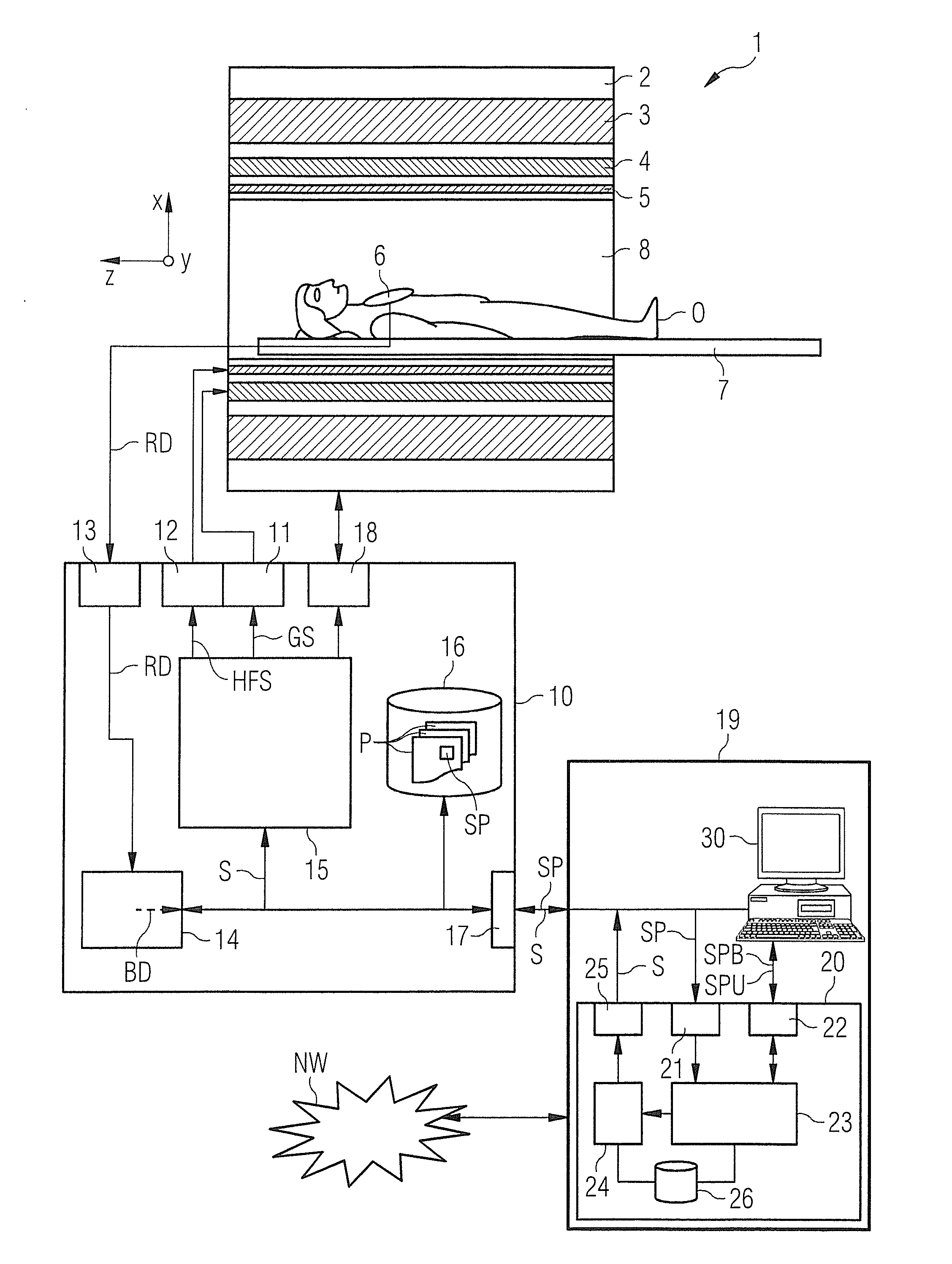
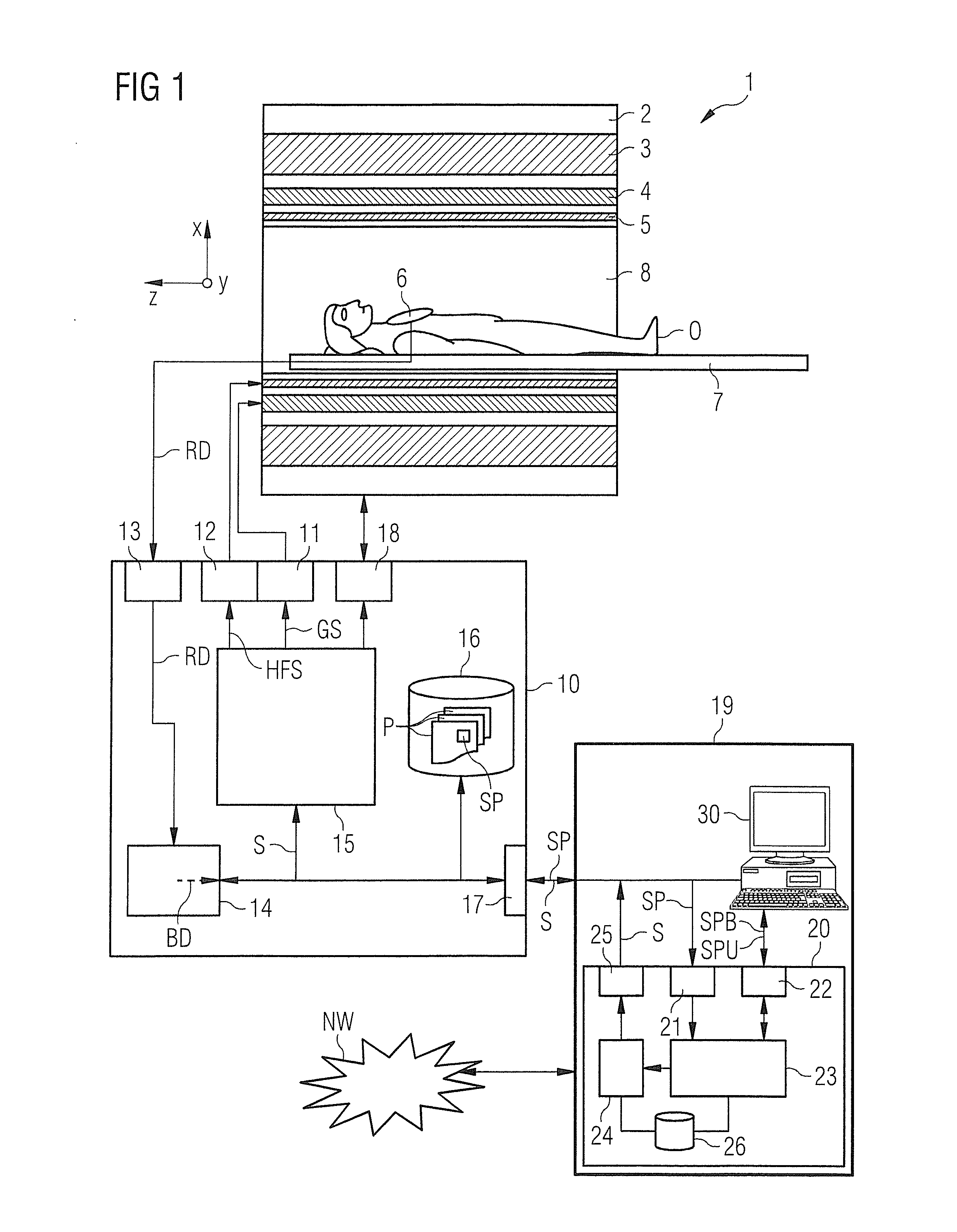
Determination of a pulse sequence for a magnetic resonance system
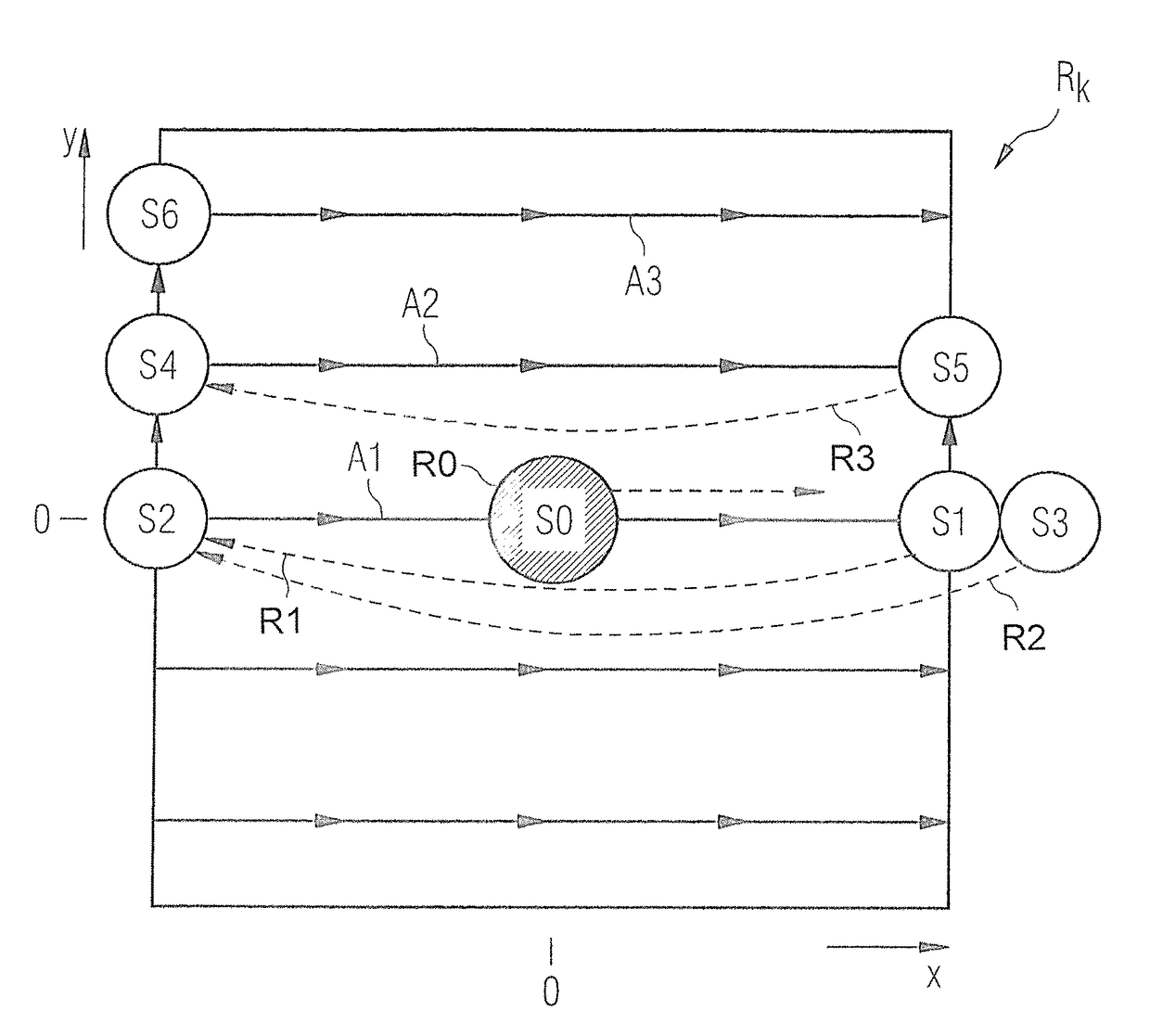
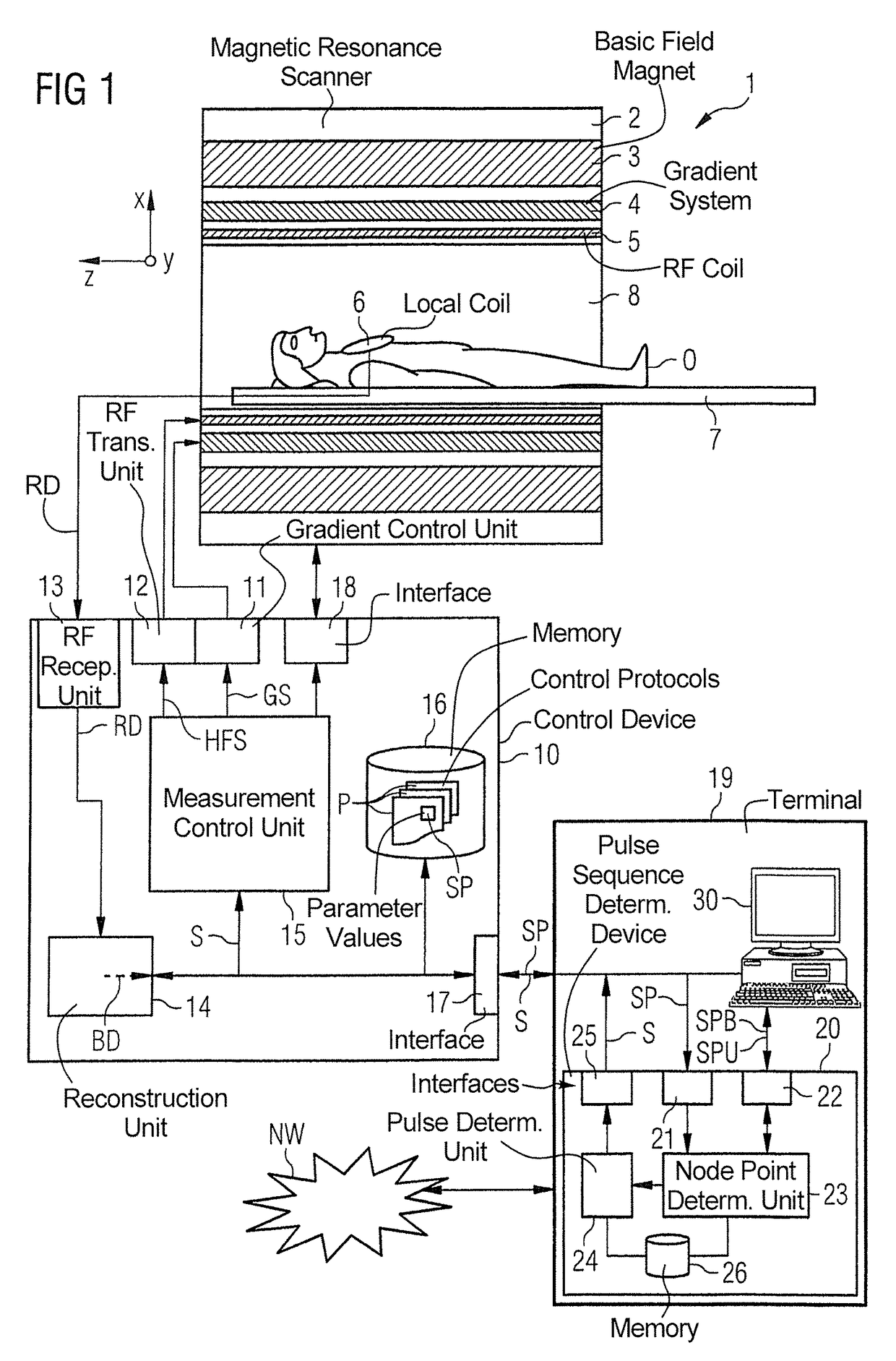
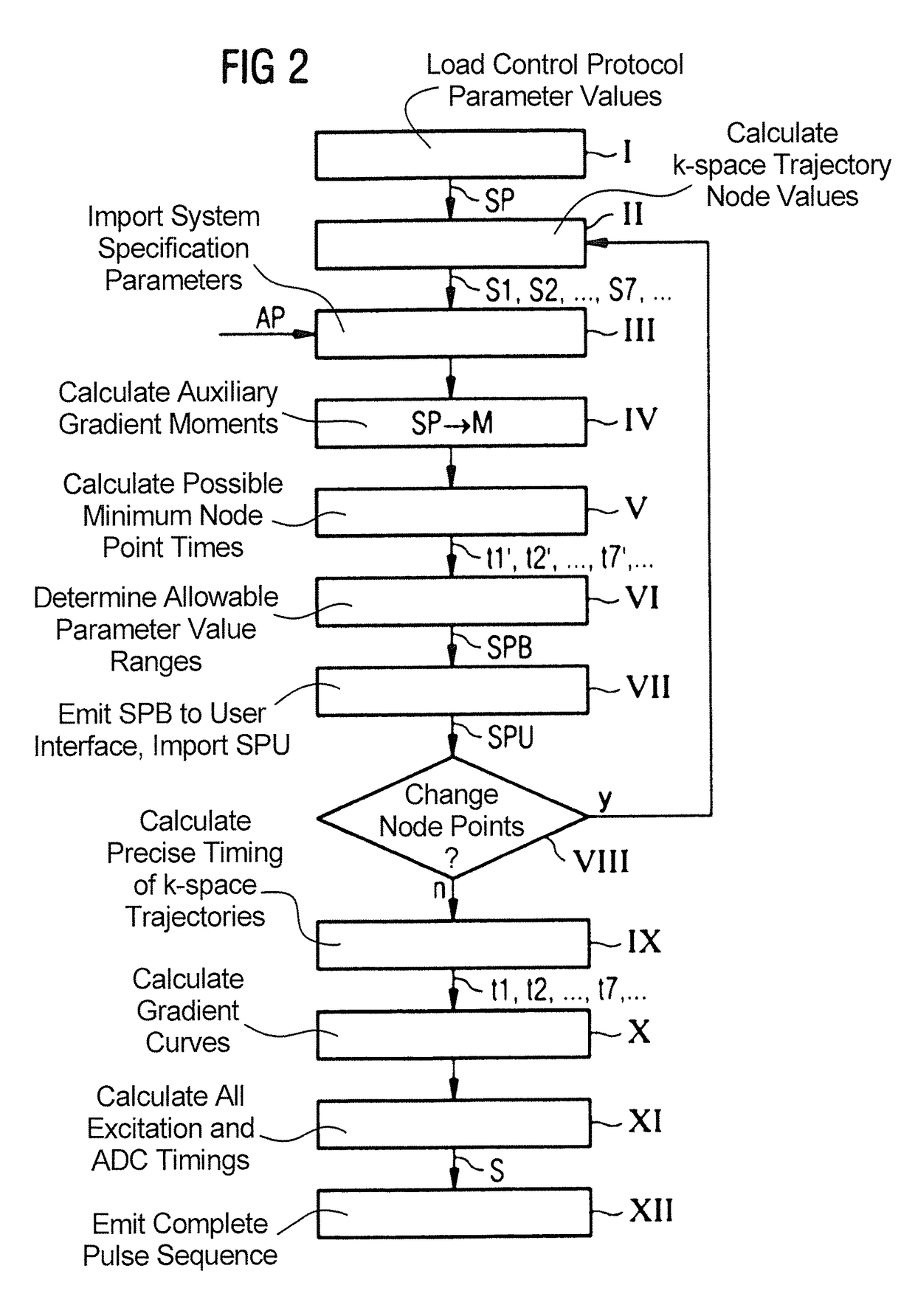
InactiveUS20140232397A1Facilitates sequence programmingLess effortMeasurements using NMR imaging systemsElectric/magnetic detectionResonanceK space trajectory
In a method and a pulse sequence determination device to determine a pulse sequence for a magnetic resonance system, control protocol parameter values are initially acquired. A determination of k-space trajectory node points within k-space then takes place in a processor on the basis of the control protocol parameter values. The determination of the pulse sequence then takes place on the basis of the k-space trajectory node points. A method for operating a magnetic resonance system uses such a pulse sequence, and a magnetic resonance system embodies such a pulse sequence determination device.
Owner:SIEMENS HEALTHCARE GMBH
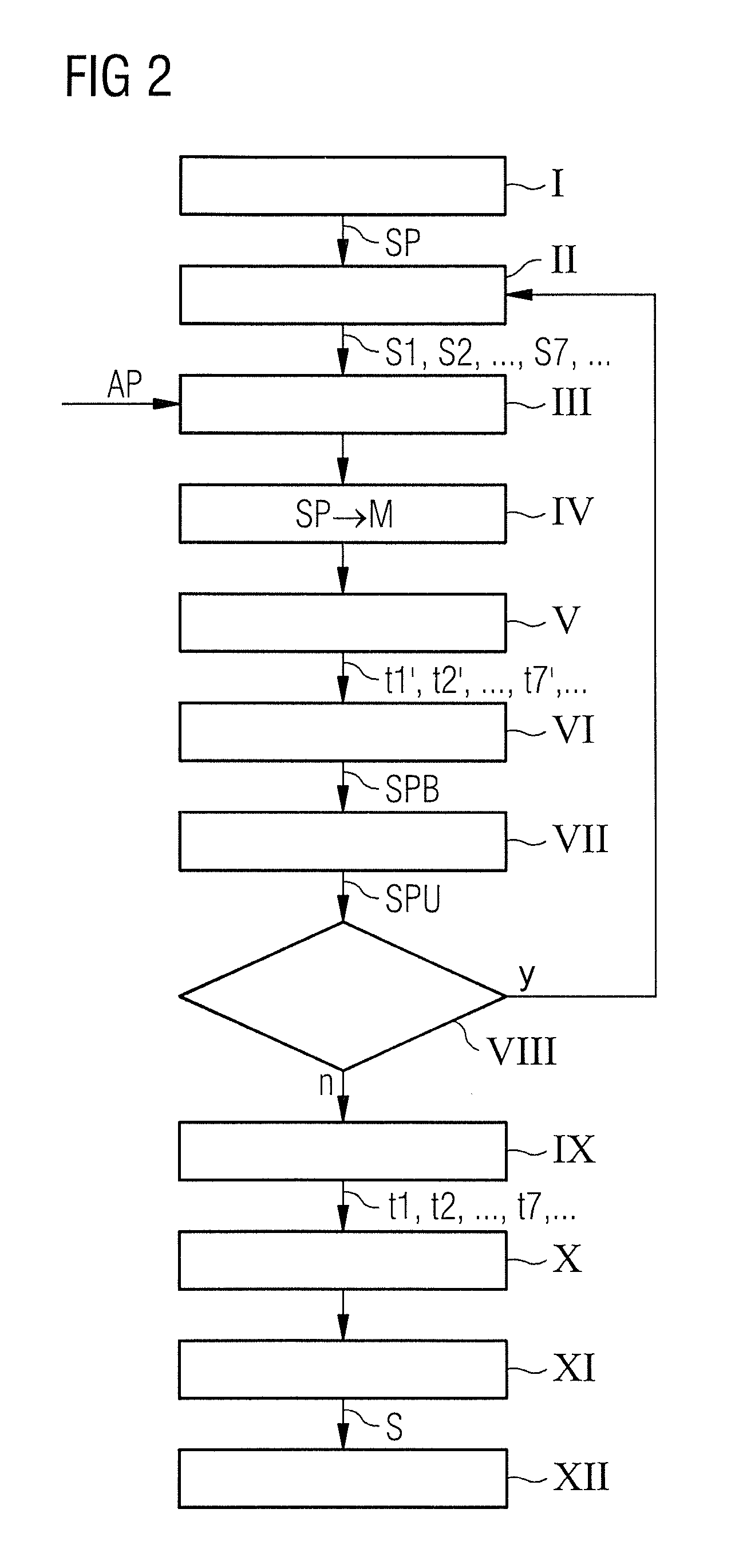
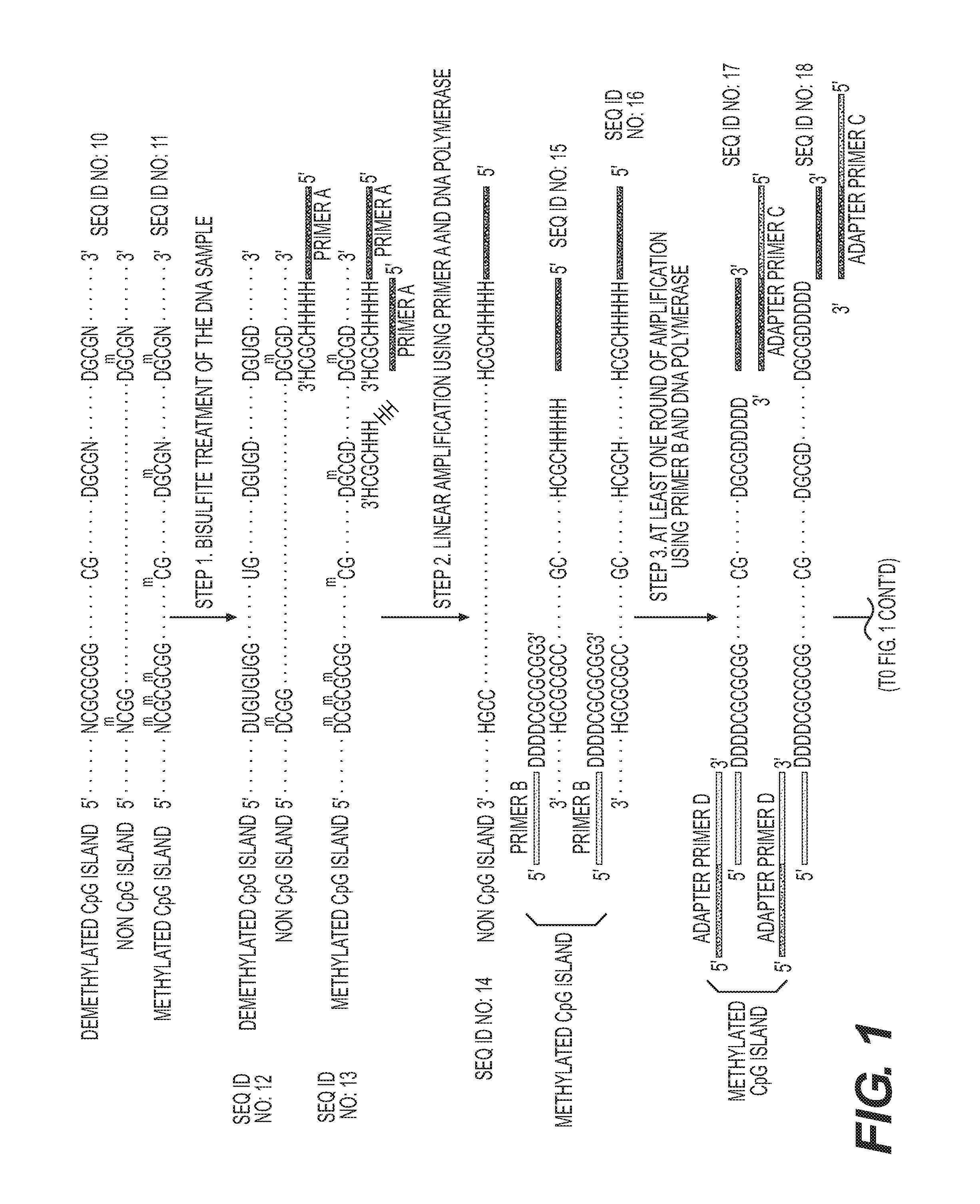
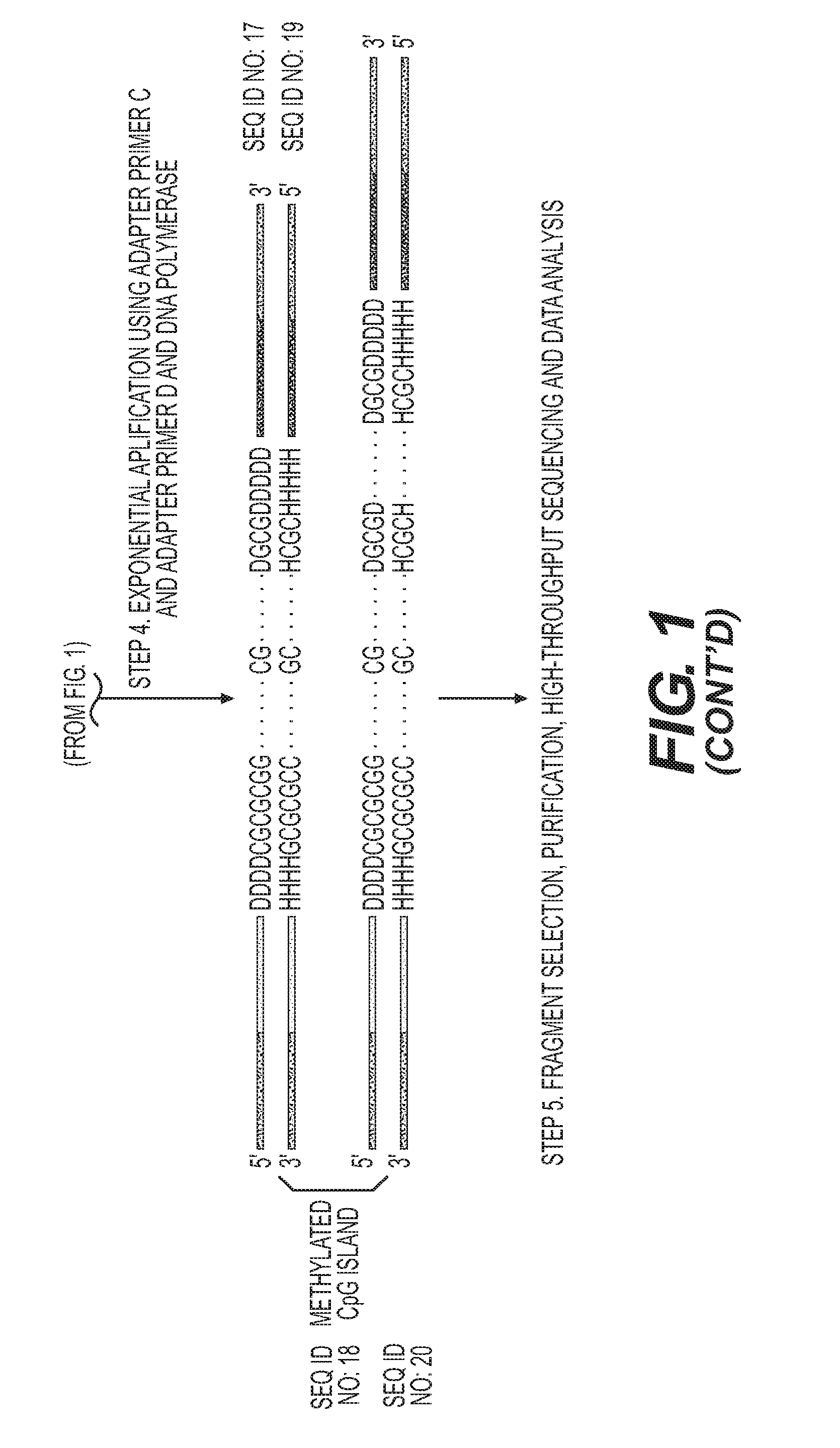
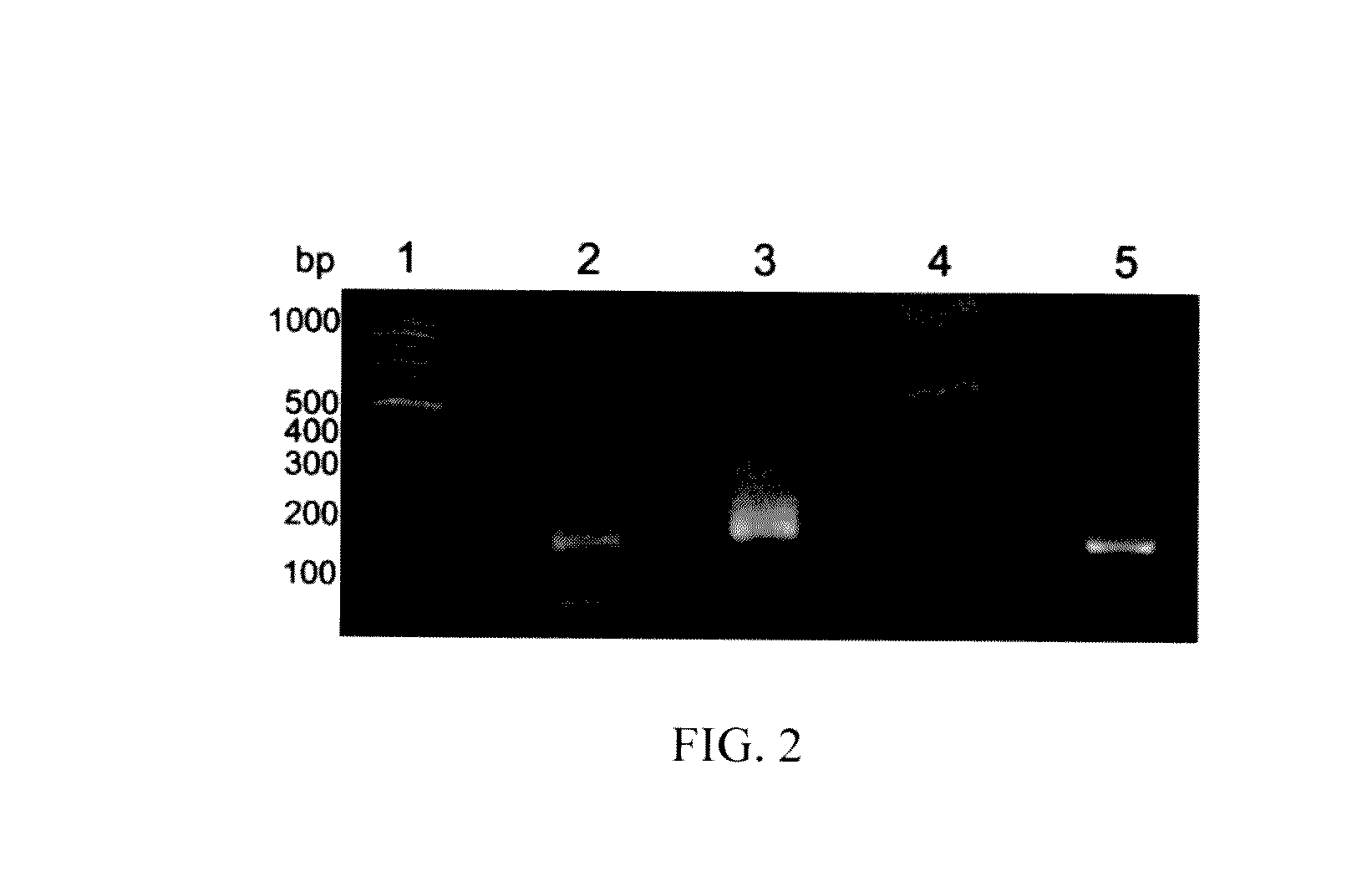
High-throughput sequencing detection method for methylated cpg islands
ActiveUS20160298183A1Efficient enrichmentCost-effectiveMicrobiological testing/measurementDna polymerasenA-DNA
A high-throughput sequencing method for detecting methylated CpG islands includes: processing a DNA sample by using a modifier, and converting cytosine in the DNA sample into uracil, and keeping 5′methylcytosine unchanged; amplifying the obtained segment by using a primer A and DNA polymerase, to obtain a segment having one end being capable of anchoring a junction primer C; amplifying the obtained segment by using a primer B and DNA polymerase, to obtain a segment gathering methylated CpG islands and having two ends being capable of separately anchoring junction primers C and D; amplifying the obtained segment at a PCR exponent by using the junction primers C and D and the DNA polymerase, to obtain the amplified product; and separating and purifying the amplified product, to form a high-throughput sequencing library and perform computer sequencing, and data analysis.
Owner:PEKING UNIV
Adhesive Patch
InactiveUS20100280467A1Easily pinch tab of upper second linerSequencing is facilitatedPlastersAdhesive dressingsMedicineBiotechnology
An adhesive patch is provided that allows the user to apply it in a safe and hygienic manner without touching the drug-containing matrix during the sequence of actions from the removal of the liner to the application of the patch. Specifically, the adhesive patch has a multilayer structure that includes a backing, an adhesive drug-containing matrix that is spread substantially entirely over one surface of the backing, and a liner that adheres to the drug-containing matrix surface. The adhesive patches have the following features:(a) the liner adhering to the drug-containing matrix surface includes first and second liners;(b) the first liner is folded at the middle thereof so that it is divided by the fold into first and second sections that together form a V-shaped liner, the first section adhering to the drug-containing matrix surface from one end of the matrix with the fold arranged closer to the middle of the drug-containing matrix, the second section serving as a tab; and(c) the second liner adheres to the remaining part of the drug-containing matrix surface with one end of the second liner covering the fold of the V-shaped first liner to form a laminated part that serves as a tab.
Owner:TEIKOKU SEIYAKU KK TEIKOKU SEIYAKU CO LTD
Backbone-modified oligonucleotide analogs and methods for using same
InactiveUS20020183502A1Improved pharmacokinetic propertiesImprove propertiesCompounds screening/testingBiocideOligomerRna expression
Therapeutic oligonucleotide analogs which have improved nuclease resistance and improved cellular uptake are provided. Replacement of phosphorodiester inter-sugar linkages found in wild type oligomers with four atom linking groups forms unique di- and poly-nucleosides and nucleotides useful in regulating RNA expression and in therapeutics. Methods of synthesis and use are also disclosed.
Owner:MESMAEKER ALAIN DE +3
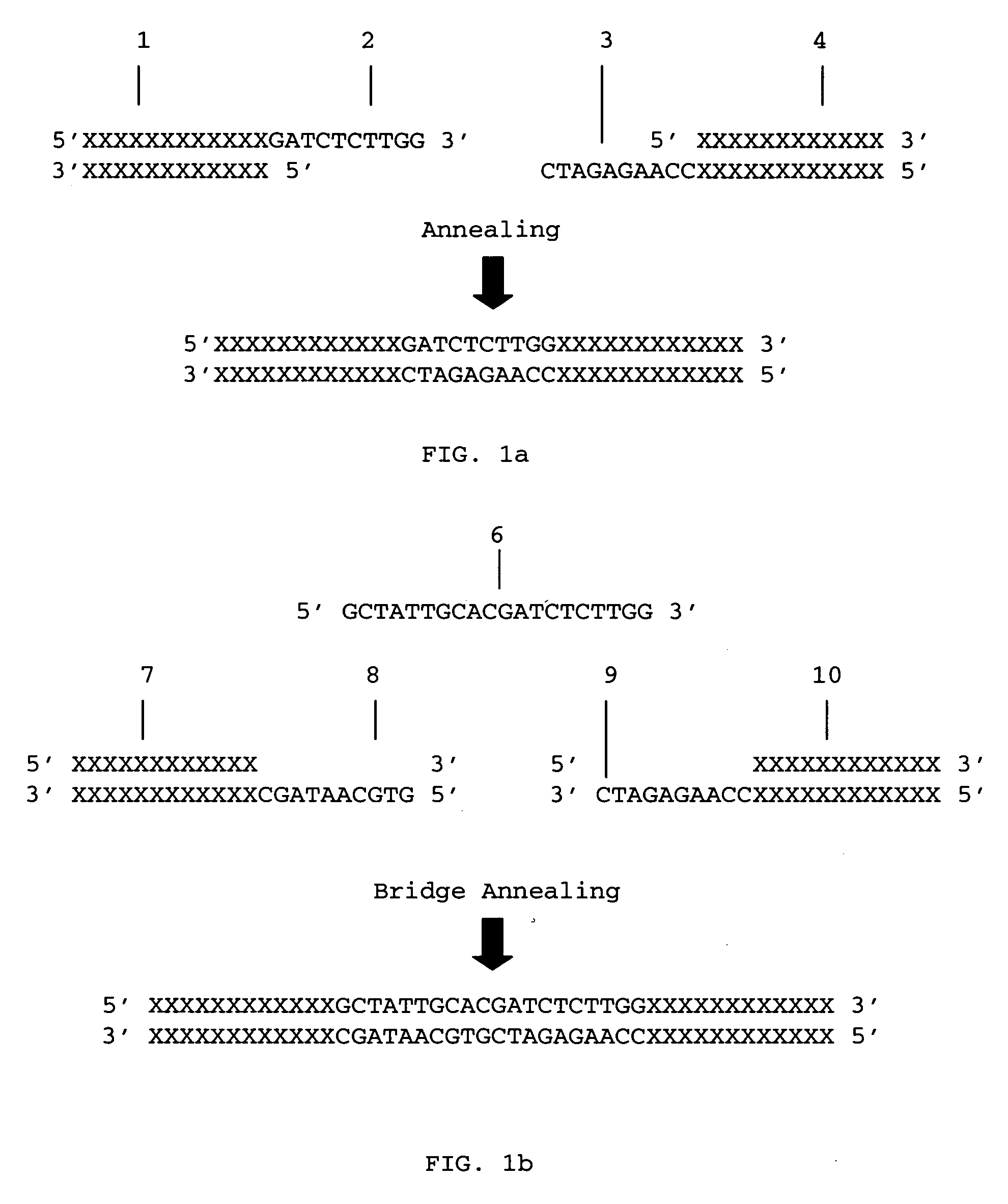
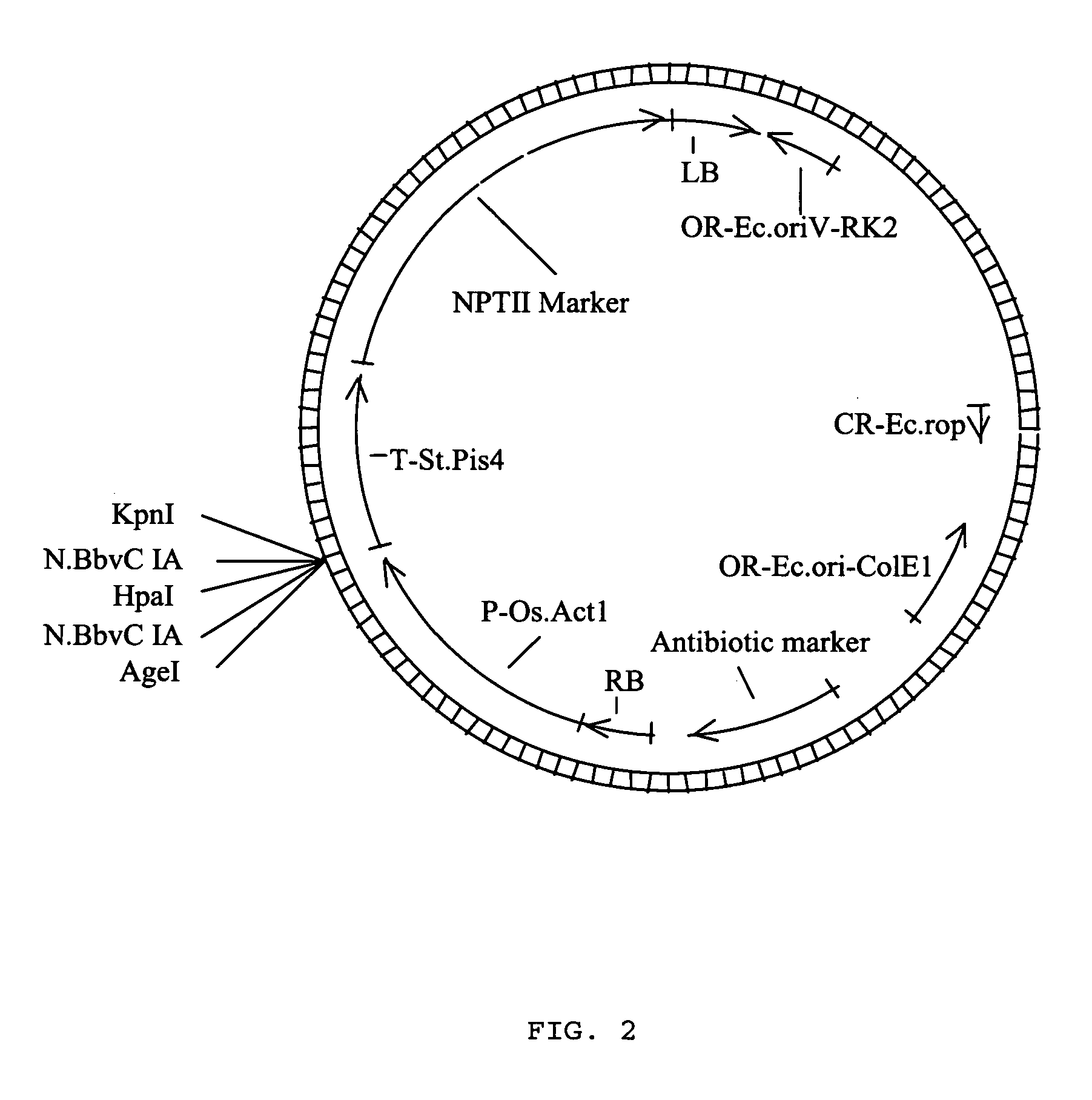
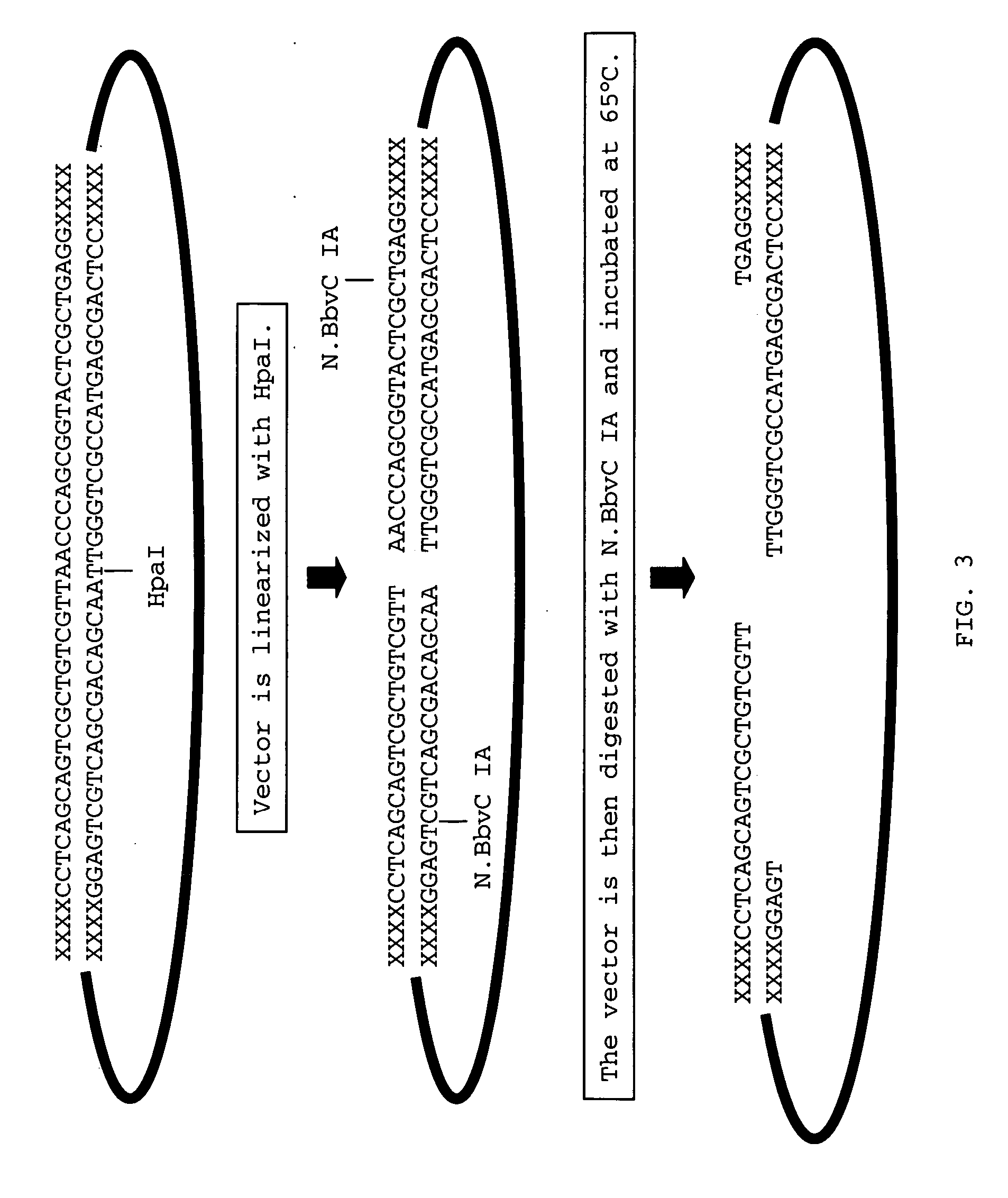
Methods for ligation independent cloning of DNA
ActiveUS20060147961A1Sequencing is facilitatedMicrobiological testing/measurementOther foreign material introduction processesBiotechnologyDNA construct
The present invention provides methods for assembling DNA molecules in a predetermined order to produce a DNA construct useful in Agrobacterium mediated transformation in plants. The method employs ligation independent cloning of separate DNA elements where one of the DNA elements contains T-DNA borders from Agrobacterium tumefaciens. The invention further provides methods for assembling a construct containing an inverted repeat. Using this approach, DNA constructs are constructed rapidly, efficiently and directionally.
Owner:MONSANTO TECH LLC
Therapeutic use of a growth factor, METRNL
ActiveUS8334264B2Promoting axonal extensionPromote migrationBiocideNervous disorderDiseaseNervous system
The present invention relates to the field of therapeutic use of proteins, genes and cells, in particular to the therapy based on the biological function of a secreted therapeutic protein, METRNL, in particular for the treatment of disorders of the nervous system. METRNL is a Nerve Survival and Growth factor with neuroprotective and / or neurogenesis effects.
Owner:HOBA THERAPEUTICS APS
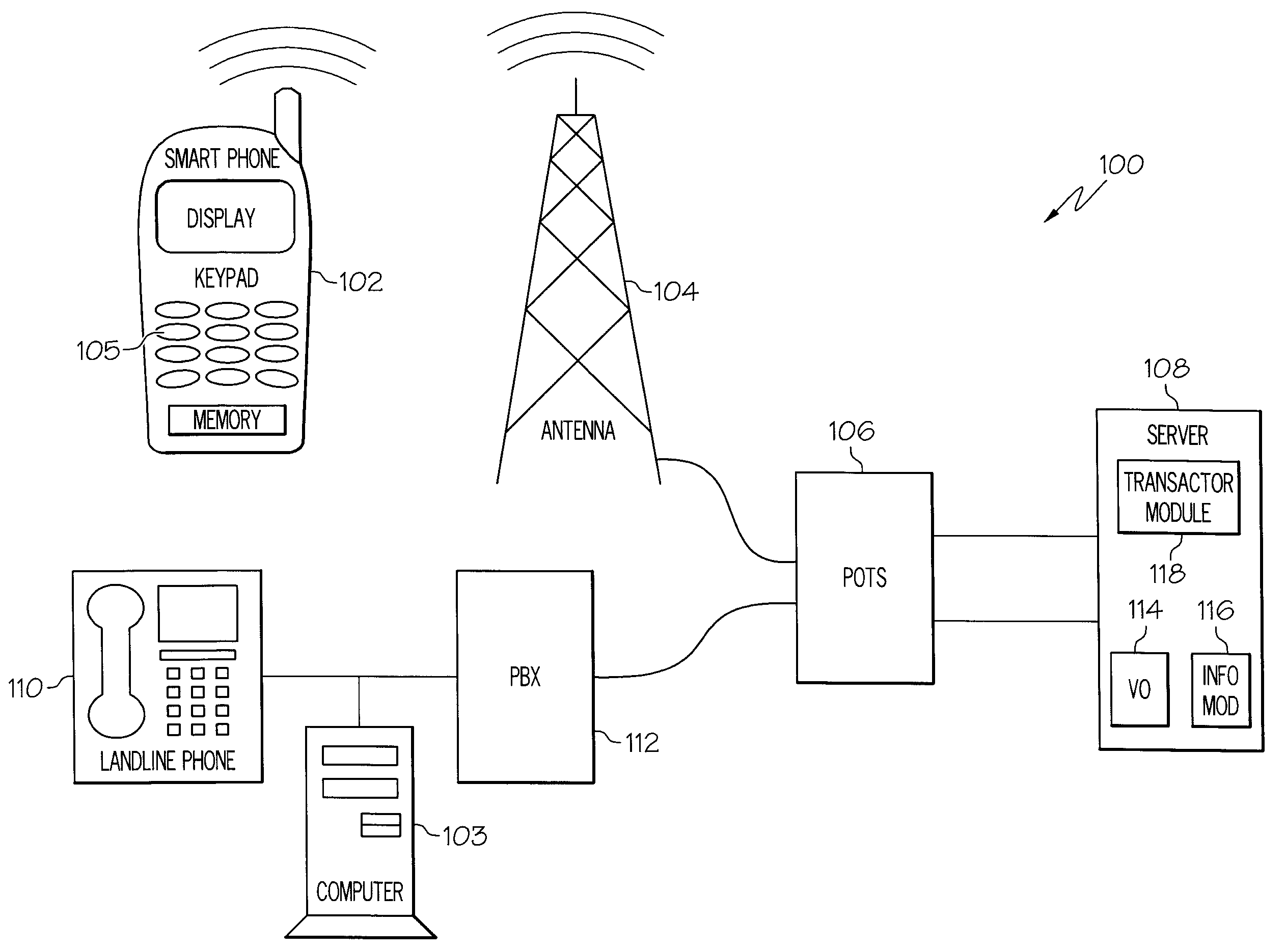
Systems and Arrangements for Communicating with an Automated Interactive Telecommunications Service System
InactiveUS20080317239A1Sequencing is facilitatedInterconnection arrangementsSubstation equipmentTelecommunications networkService system
In one embodiment a method for accessing and interacting with an interactive telecommunications service system is disclosed. The method can include activating a programming mode, entering a plurality of keystrokes to create a keyed sequence where the keyed sequence can include a telephone number and a menu response sequence. The menu response sequence can control the menu driven portion of the telecom service system after the device is connected via a telecommunications network. The keyed sequence can include delays between each of the plurality of keystrokes. The delays can be recorded and a session identifier can also be recorded. The keyed sequence can be associated with the delays and the session identifier in response to the activated programming mode. Access to the service and results from a service request can be automated by the disclosed arrangements.
Owner:KYNDRYL INC
Genomic selection and sequencing using encoded microcarriers
ActiveUS20110311975A1Add information contentShorten the timeBioreactor/fermenter combinationsBiological substance pretreatmentsNucleotideA-DNA
The present invention relates to a method for determining the sequence of a nucleic molecule. Herein a capture oligonucleotide probe is attached to an encoded microcarrier, wherein the code of said microcarrier identifies the sequence of said oligonucleotide probe. The capture oligonucleotide probe is hybridized with a sample comprising nucleic acids molecules, wherein said DNA fragment comprises a sequence which is complementary to the sequence of the capture oligonucleotide probe. The sequence of the DNA molecule is determined, wherein the capture oligonucleotide probe serves as a primer for a DNA polymerase, in the case of single molecule sequencing this is a sequencing primer. After the sequence determination, the nucleotide sequence of the capture oligonucleotide probe is identified by determining the code on the microcarrier, which corresponds with the capture oligonucleotide probe. This sequence information directly identifies the location of the sequenced DNA fragment on the genome, allowing direct comparison.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Shoe sole with foot guidance
A sole for a shoe with a foot guiding mechanism which has the particularity that the sole comprises a sole body which has, on an outer face thereof, at least one protrusion. The at least one protrusion is flexible in order to produce a desired movement of the protrusion which is suitable to force a guided sequence for a foot, wearing the shoe sole, from when the heel section initially contacts a ground surface to when the front edge of the sole breaks contact with the ground surface.
Owner:ALINE SYST INC
Oligonucleotide mimics having nitrogen-containing linkages
InactiveUS6271357B1Sequencing is facilitatedImprove propertiesGroup 4/14 element organic compoundsSugar derivativesNitrogenOligonucleotide
Owner:IONIS PHARMA INC
Dominant negative mutant KRP protein protection of active cyclin-CDK complex inhibition by wild-type KRP
InactiveUS8742205B2Maintaining binding affinity for a cyclin proteinDominant negative antagonist activitySugar derivativesBacteriaCell divisionMutated protein
Disclosed are mutant CDK inhibitor (CKI) polypeptides having dominant negative antagonist activity against wild-type CKI proteins, as well as related compositions, including nucleic acids and vectors encoding the mutant CKI polypeptides and transformed host cells and transgenic plants comprising such nucleic acids and vectors. Also disclosed are related methods for using the mutant proteins to modulate cell division in cells, particularly plant cells.
Owner:TARGETED GROWTH INC
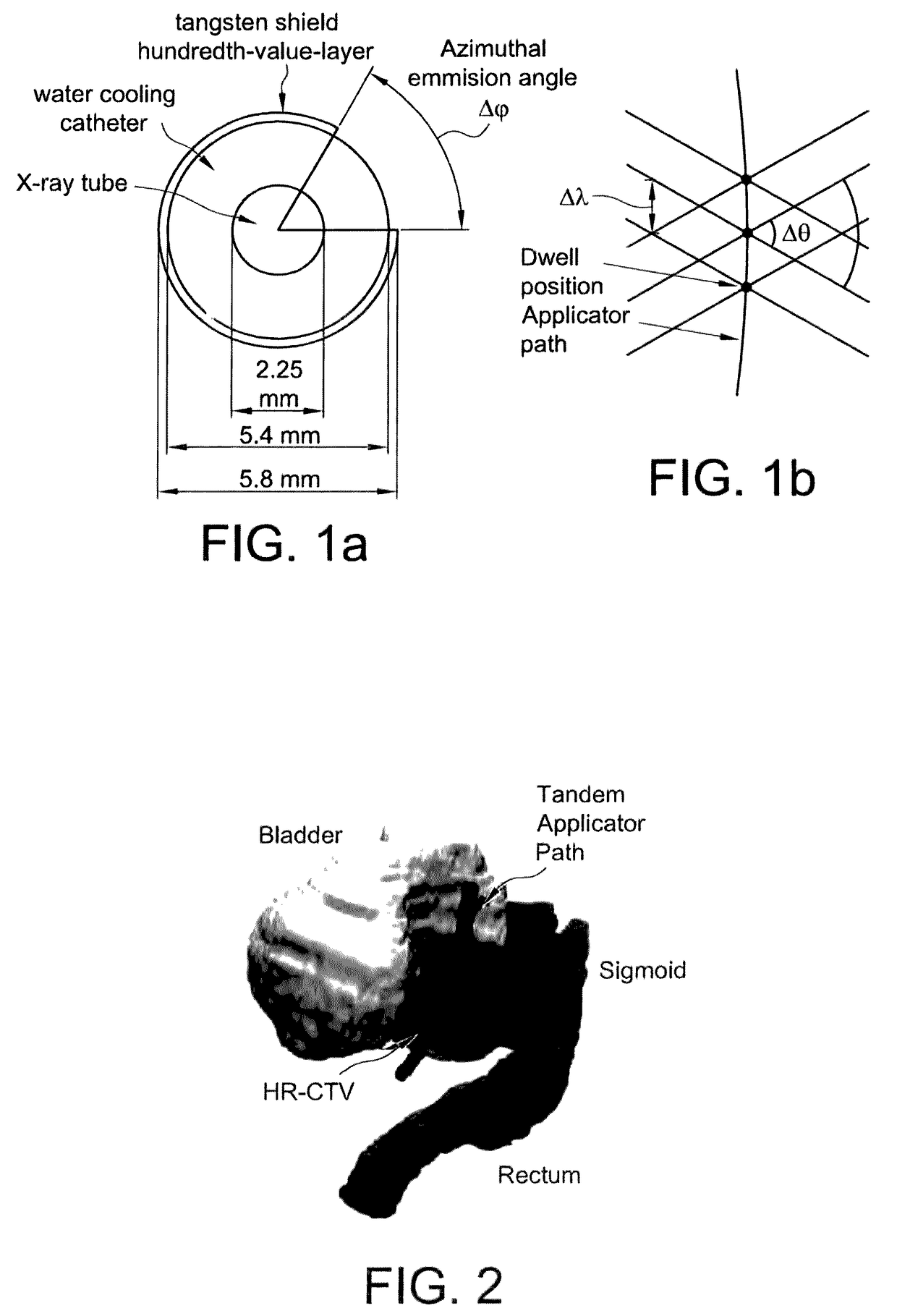
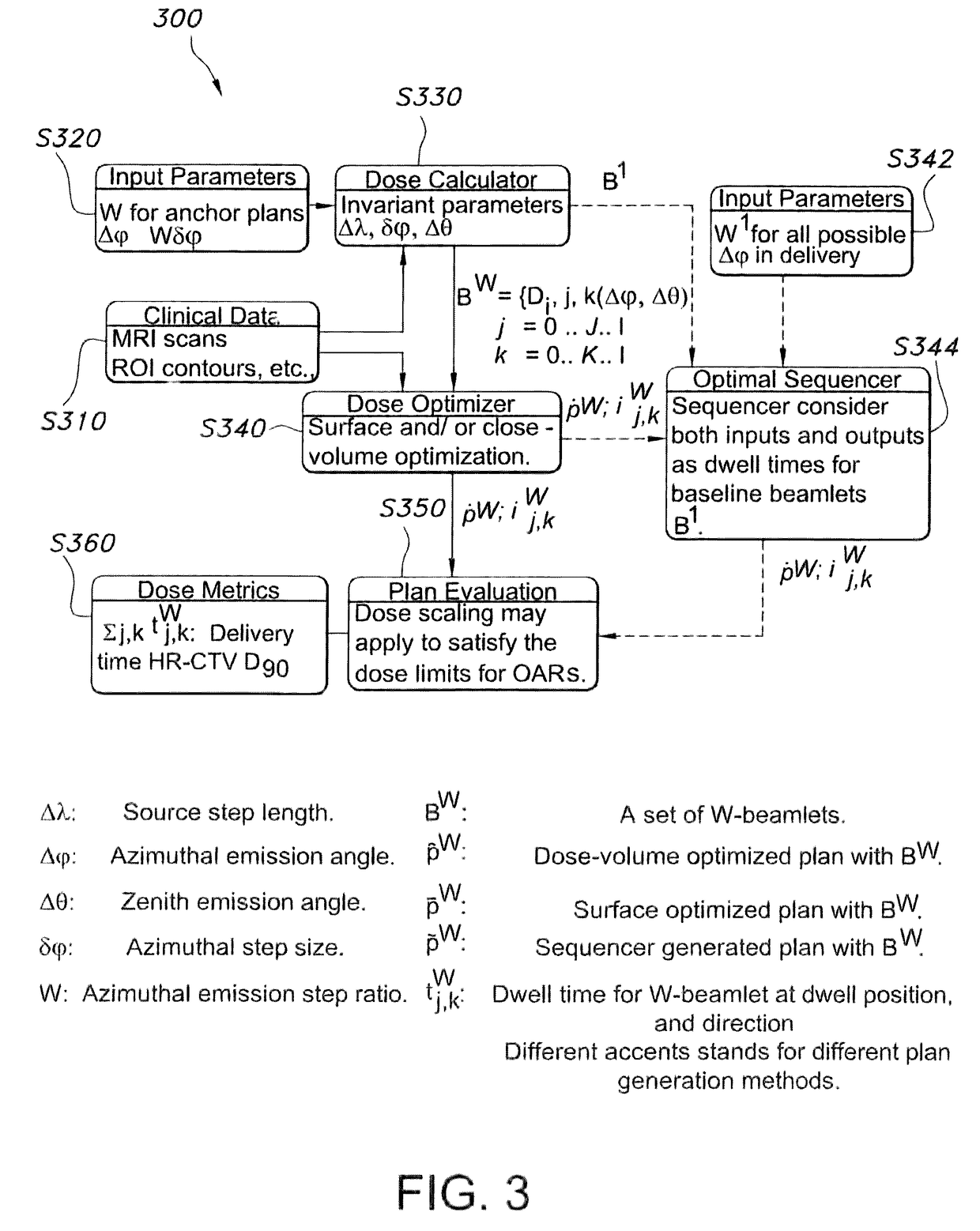
Advanced rotating-shield brachytherapy and planning of the same
ActiveUS20170165500A1Reduce planning timeImprove consistencyX-ray/gamma-ray/particle-irradiation therapyBrachytherapyEngineering
Systems and methods for rotating shield brachytherapy. In an aspect, some of the systems and methods can be used to facilitate shield selection for use in rotating shield brachytherapy. In an aspect, the invention is a shielded needle or catheter system with a rotational controller for delivering radioisotope-based interstitial rotating shield brachytherapy (I-RSBT). In an aspect, the catheter system can utilize paddle-based RSBT. Further provided are methods and systems for helical RSBT.
Owner:UNIV OF IOWA RES FOUND
Determination of a pulse sequence for a magnetic resonance system
InactiveUS9632160B2Less effortSequencing is facilitatedMeasurements using NMR imaging systemsResonanceK space trajectory
In a method and a pulse sequence determination device to determine a pulse sequence for a magnetic resonance system, control protocol parameter values are initially acquired. A determination of k-space trajectory node points within k-space then takes place in a processor on the basis of the control protocol parameter values. The determination of the pulse sequence then takes place on the basis of the k-space trajectory node points. A method for operating a magnetic resonance system uses such a pulse sequence, and a magnetic resonance system embodies such a pulse sequence determination device.
Owner:SIEMENS HEALTHCARE GMBH
Method of MR (=magnetic resonance) with spatial encoding to generate an image or spectroscopic data
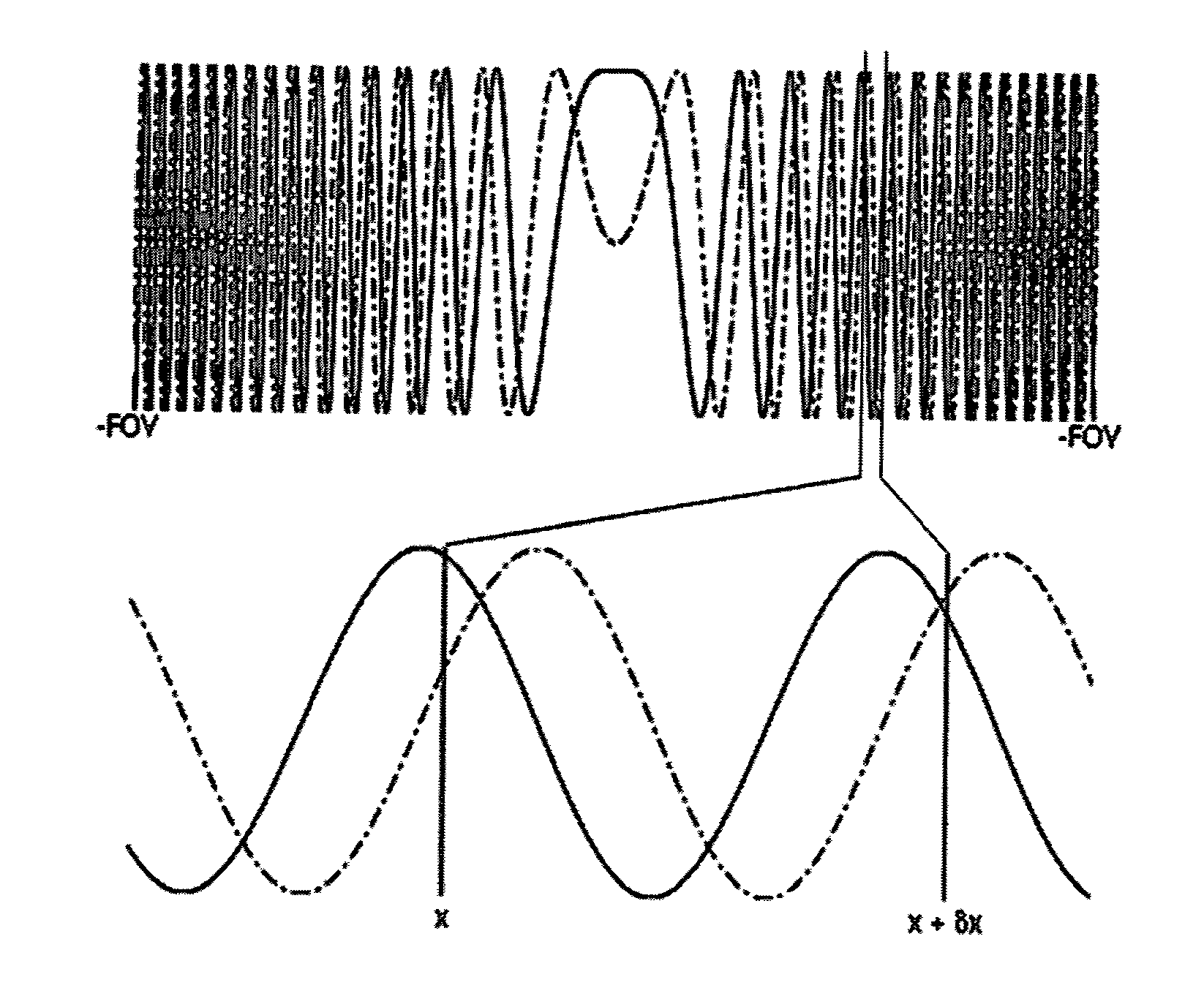
InactiveUS8791700B2Sequencing is facilitatedImprove signal-to-noise ratioElectric/magnetic detectionMeasurements using NMRResearch ObjectTransverse magnetization
A method of MR with spatial encoding to generate an image or spectroscopic data of an object of investigation inside an MR apparatus comprises the steps of (a) selecting a volume of interest within the object of investigation, (b) applying an RF pulse to generate a transverse magnetization within the object of investigation, (c) preparing a nonlinear phase distribution within the object of investigation by application of spatially encoding magnetic fields (SEMs), the SEMs comprising of a nonlinear gradient field or a combination of linear and nonlinear gradient fields, (d) effecting primary spatial encoding through application of SEMs, and (e) recording MR signals originating from the object of investigation. Step (c) or (d) thereby comprises applying a sequence of at least two SEMs, at least one of which contains a nonlinear field gradient and at least two of the SEMs having different field geometries. The sequence of SEMs is applied at a point in time from and including the excitation of the object of interest in step (b) up to and including the recording of the MR signals in step (e), to thereby introduce a temporal shift of the signals arising from spatially different locations within the selected volume of interest, that is to thereby introduce a shift of local spatial frequency components. A sampling window for recording of the respective MR signals is set and signals originating from the volume of interest are recorded in step (e) and undesired signals originating from outside the volume of interest are suppressed.
Owner:UNIVERSITATSKLINIKUM FREIBURG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com