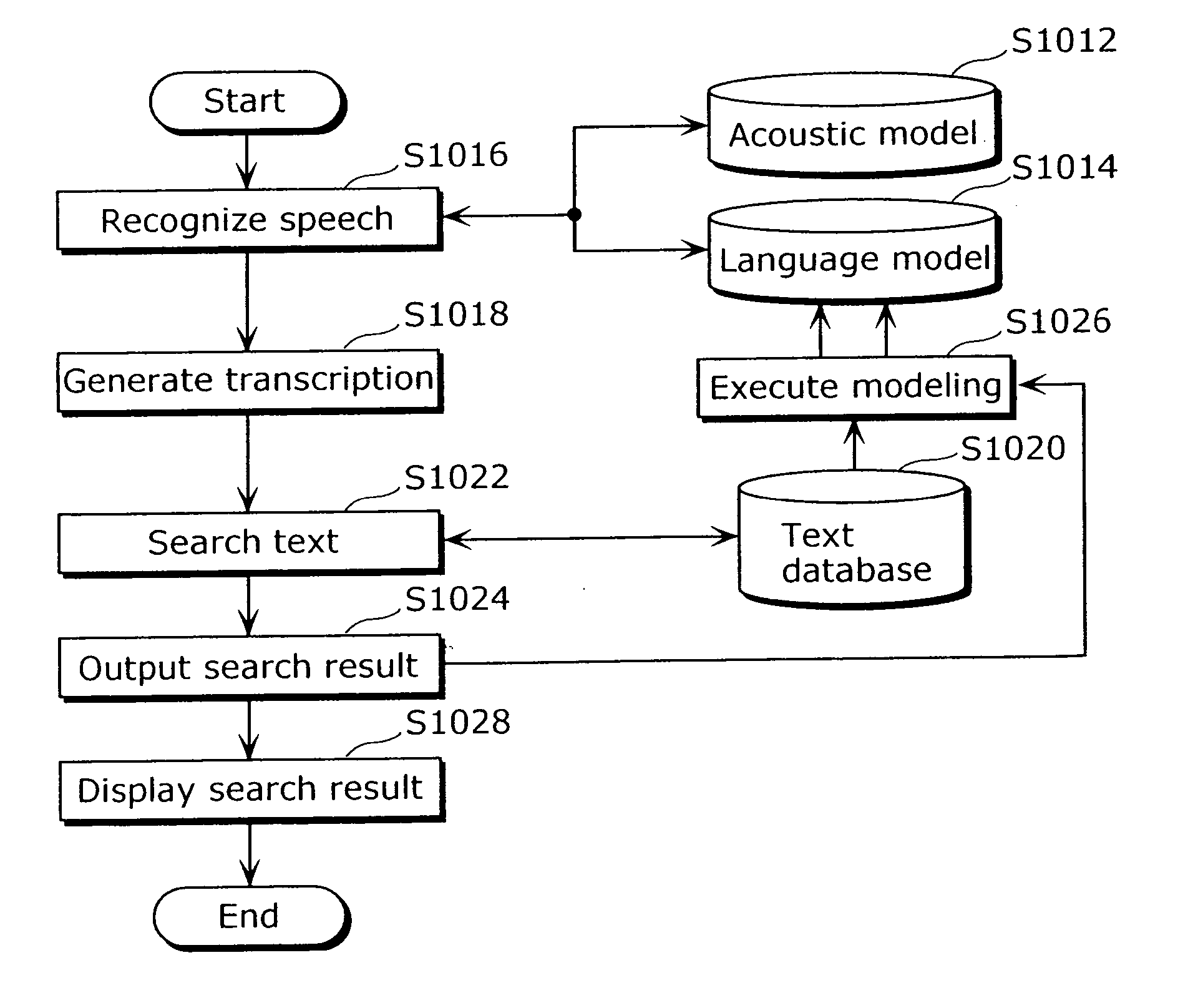
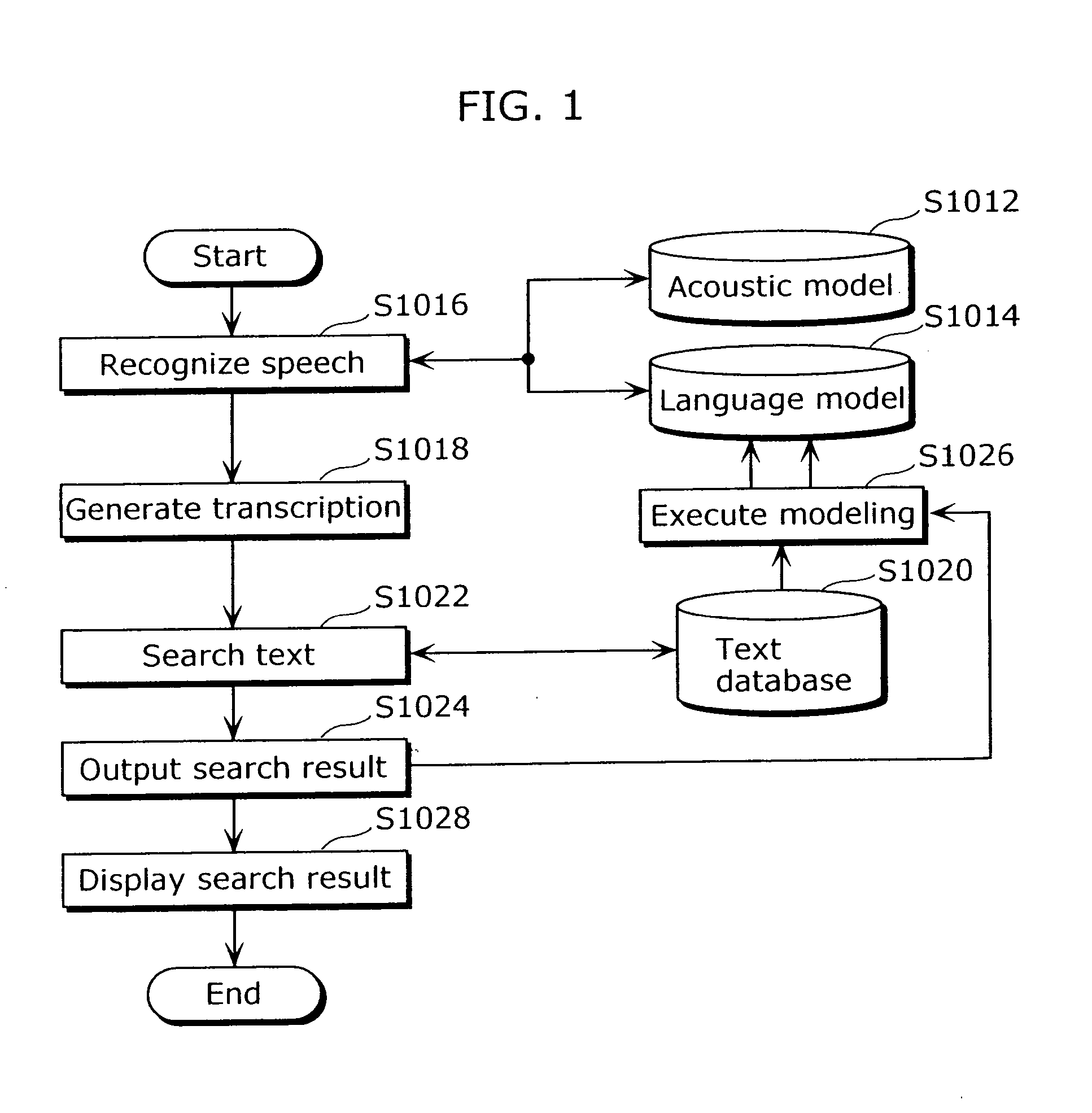
Patents
Literature
477results about How to "Complicated process" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Personal choice biometric signature
InactiveUS7013030B2Improved high security method and systemEasy to useElectric signal transmission systemsImage analysisCombined useLinearity
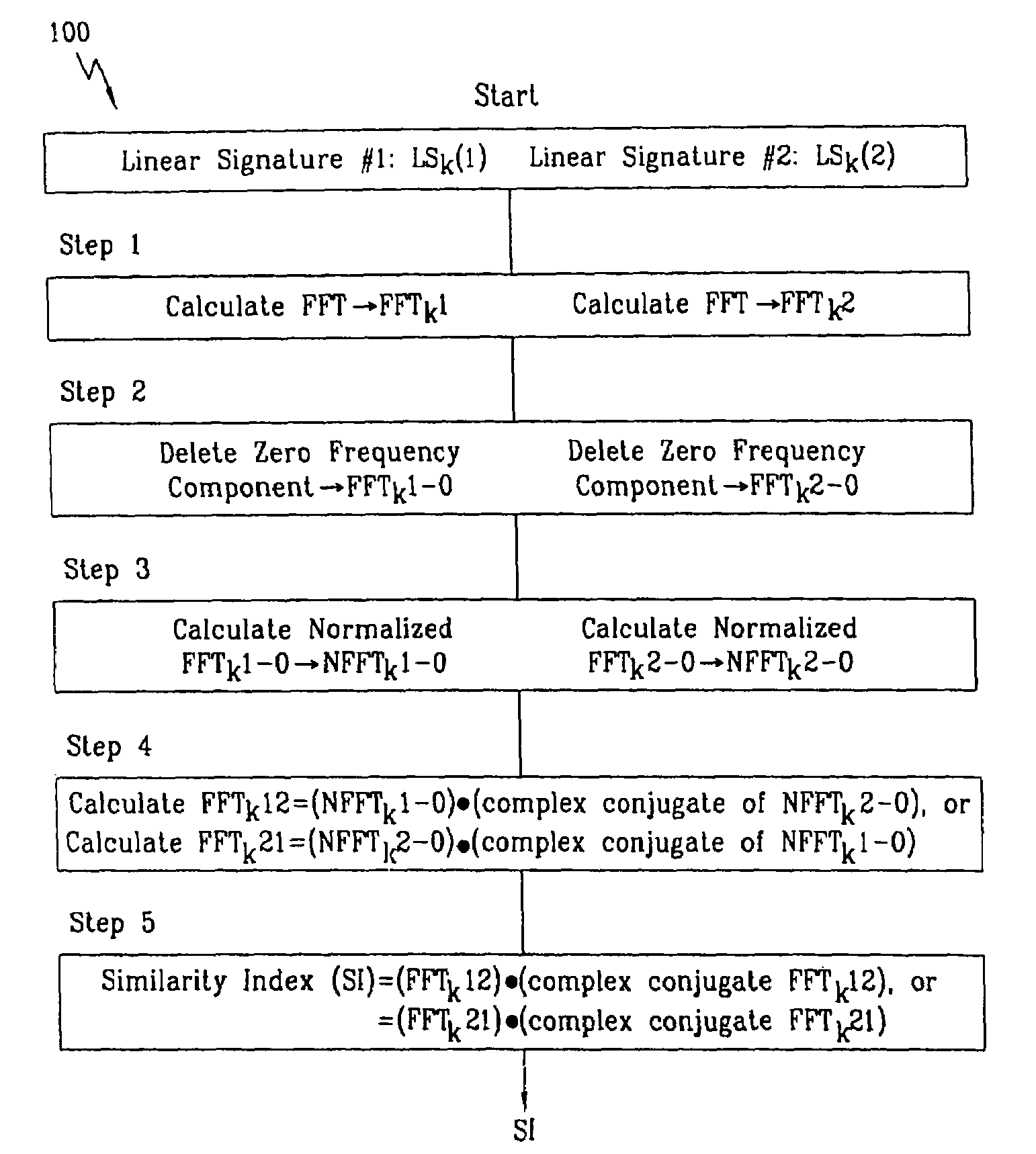
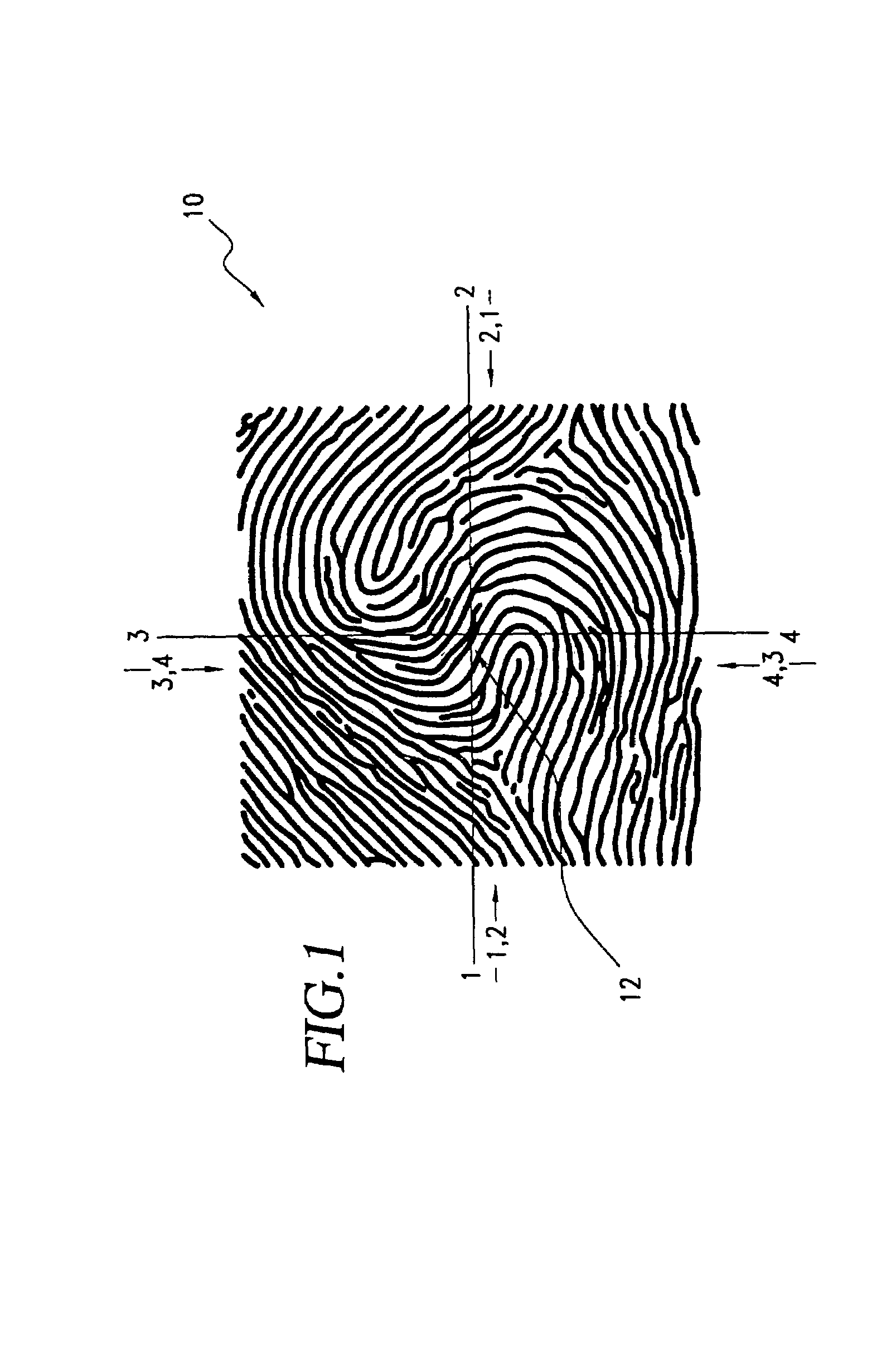
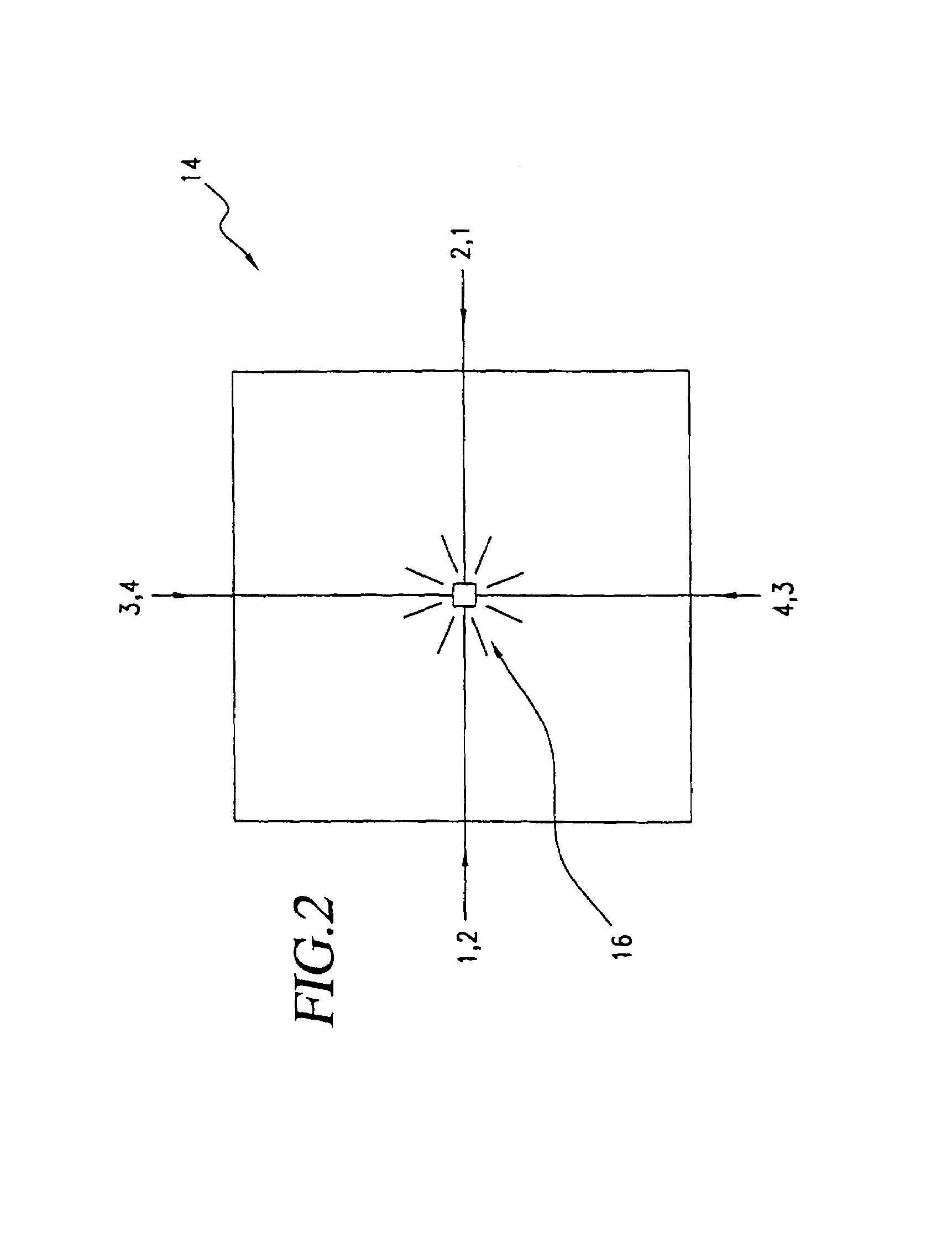
A biometric method and system for personal authentication using sequences of partial fingerprint signatures provides a high security capability to various processes requiring positive identification of individuals. This approach is further enhanced by employing a frequency domain technique for calculating a Similarity Index of the partial fingerprint signatures. In a baseline usage, the sequential partial fingerprint sequence techniques augments sentinel systems for gaining access to restricted areas, and when used in combination with financial cards, offer a unique and greatly simplified means for authenticating or identifying individuals. A highly automated technique initially obtains four (illustratively) linear partial fingerprint signatures which serve as reference data against which later proffered candidate data in the form of at least two linear partial fingerprint signatures are compared for authentication. The particular two candidate signatures used and the sequence in which they are submitted are selected with the user's consent and serve as a PIN-like unique personal code. In an advanced embodiment, the same two candidate signatures in the chosen sequence are processed in a unique FFT / DFT process to produce a highly reliable Similarity Index to authenticate or verify the identity of individuals. The use of only partial fingerprint data greatly allays the concerns of widespread fingerprint dissemination by many individuals.
Owner:WONG JACOB Y +1
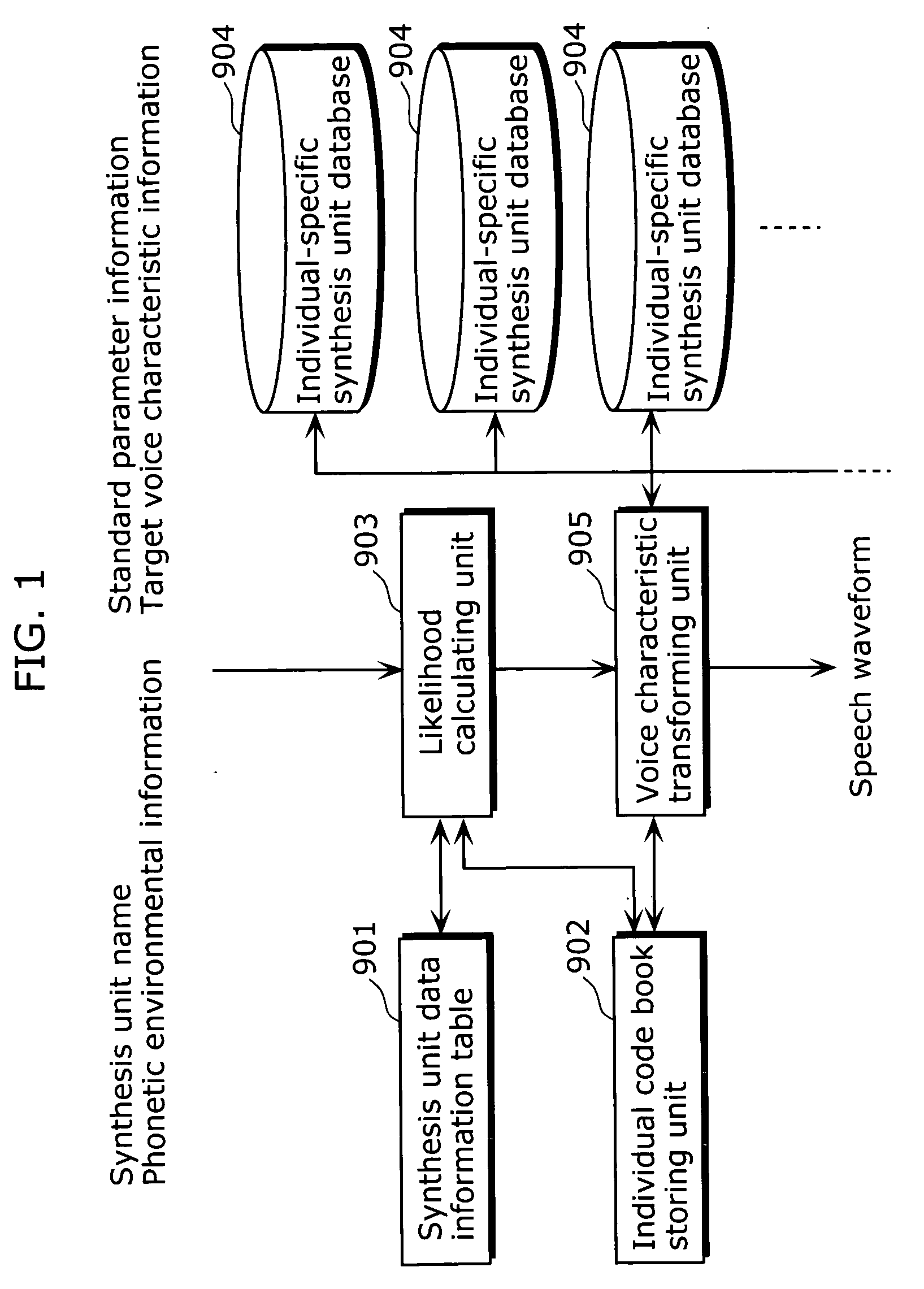
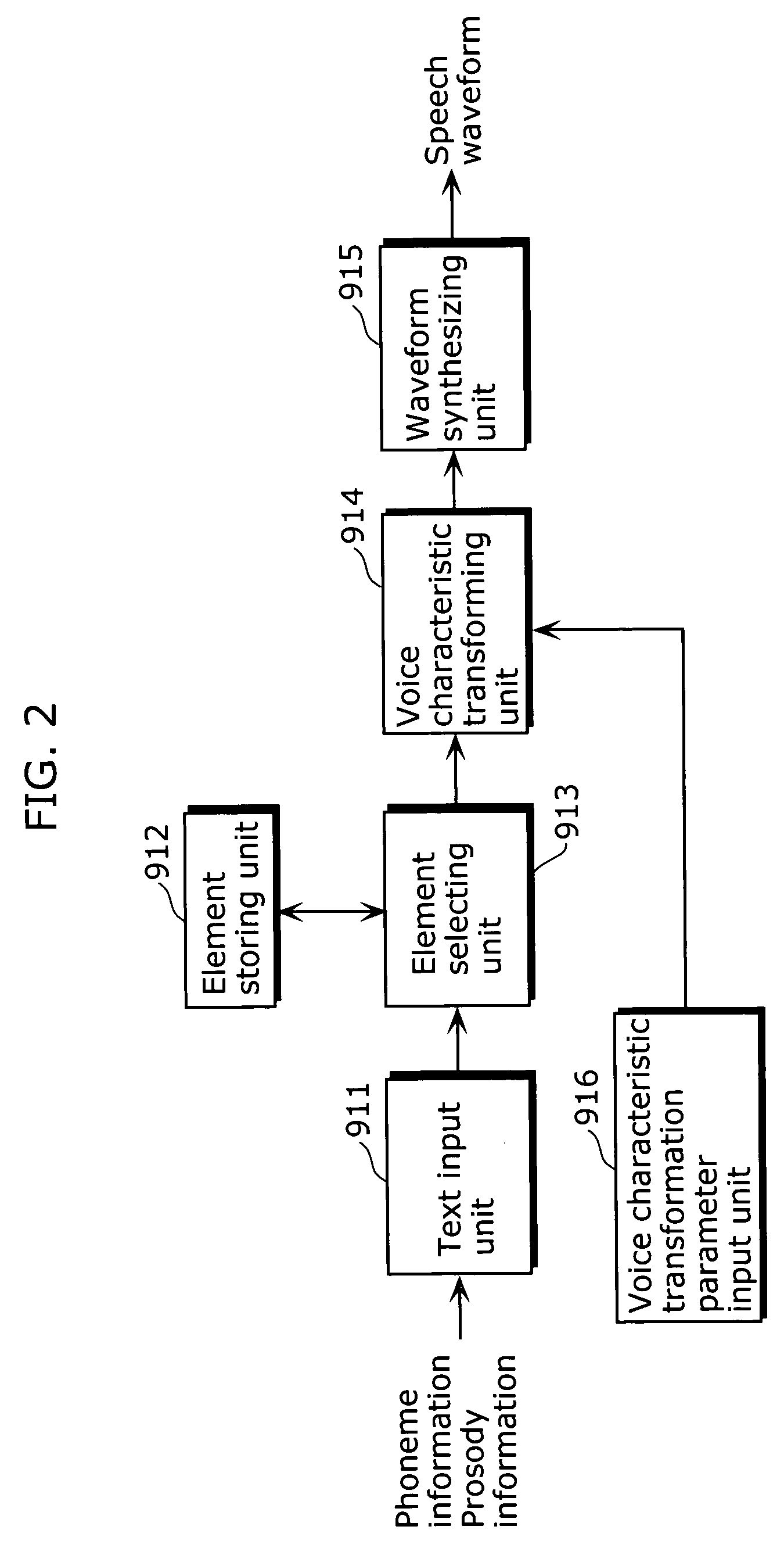
Speech synthesis apparatus and speech synthesis method
ActiveUS20060136213A1Appropriately transformedQuality improvementSpeech synthesisAcousticsSpeech synthesis
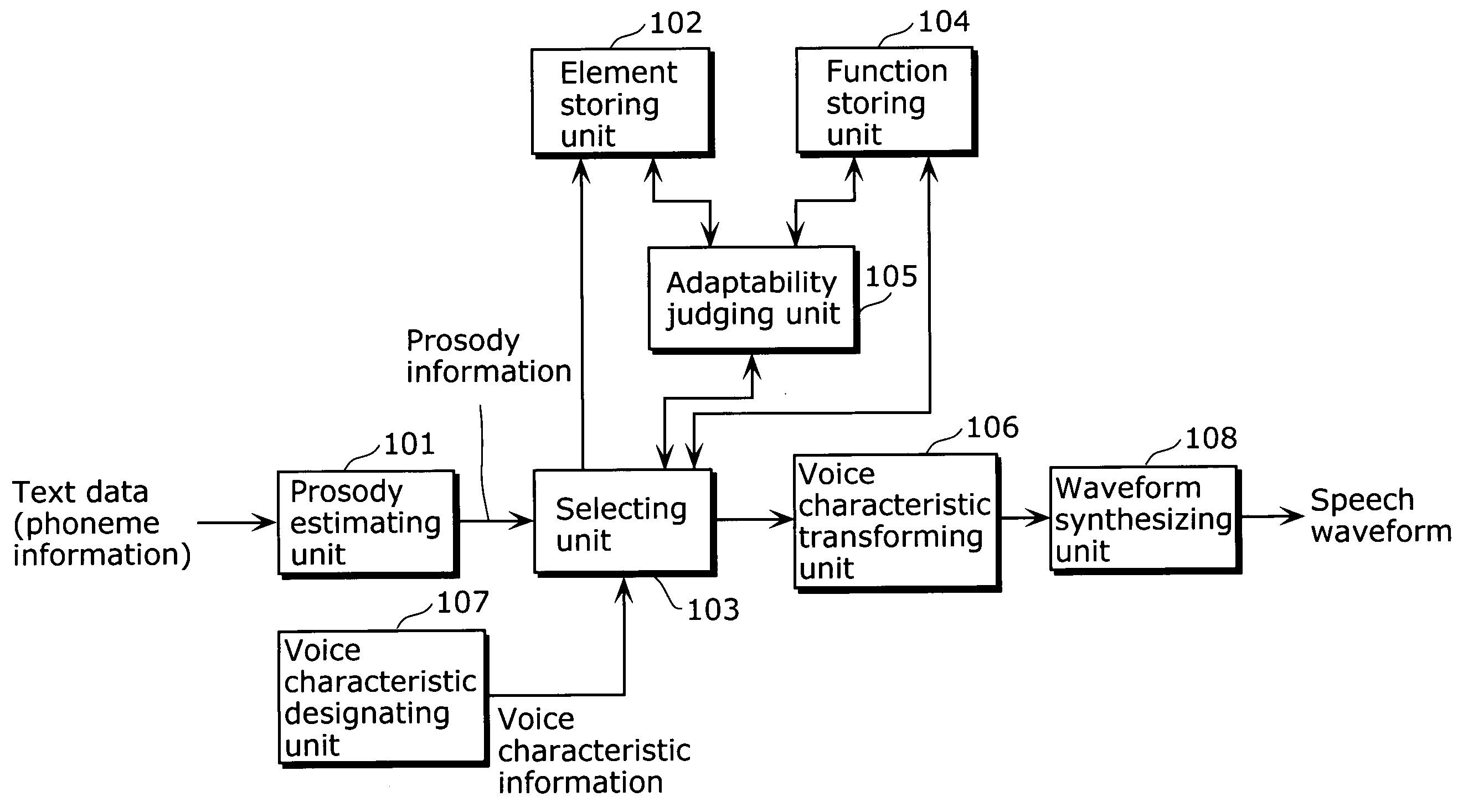
A speech synthesis apparatus which can appropriately transform a voice characteristic of a speech is provided. The speech synthesis apparatus includes an element storing unit in which speech elements are stored, a function storing unit in which transformation functions are stored, an adaptability judging unit which derives a degree of similarity by comparing a speech element stored in the element storing unit with an acoustic characteristic of the speech element used for generating a transformation function stored in the function storing unit, and a selecting unit and voice characteristic transforming unit which transforms, for each speech element stored in the element storing unit, based on the degree of similarity derived by the adaptability judging unit, a voice characteristic of the speech element by applying one of the transformation functions stored in the function storing unit.
Owner:PANASONIC INTELLECTUAL PROPERTY CORP OF AMERICA
Method of growing electrical conductors
ActiveUS7067407B2Quality improvementGood step coverageSemiconductor/solid-state device manufacturingCapacitorsElectrical conductorAtomic layer deposition
Owner:ASM INTERNATIONAL
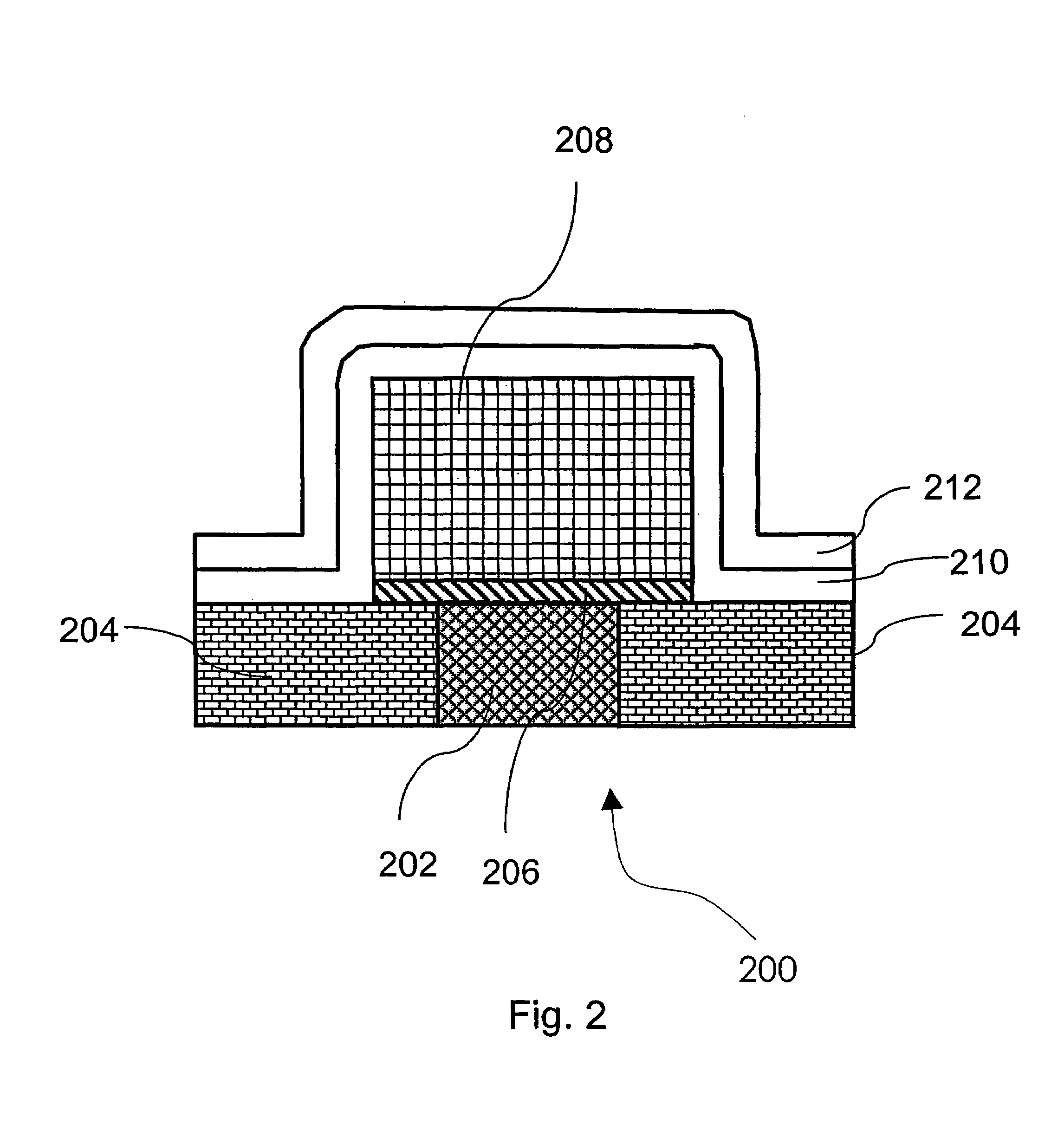
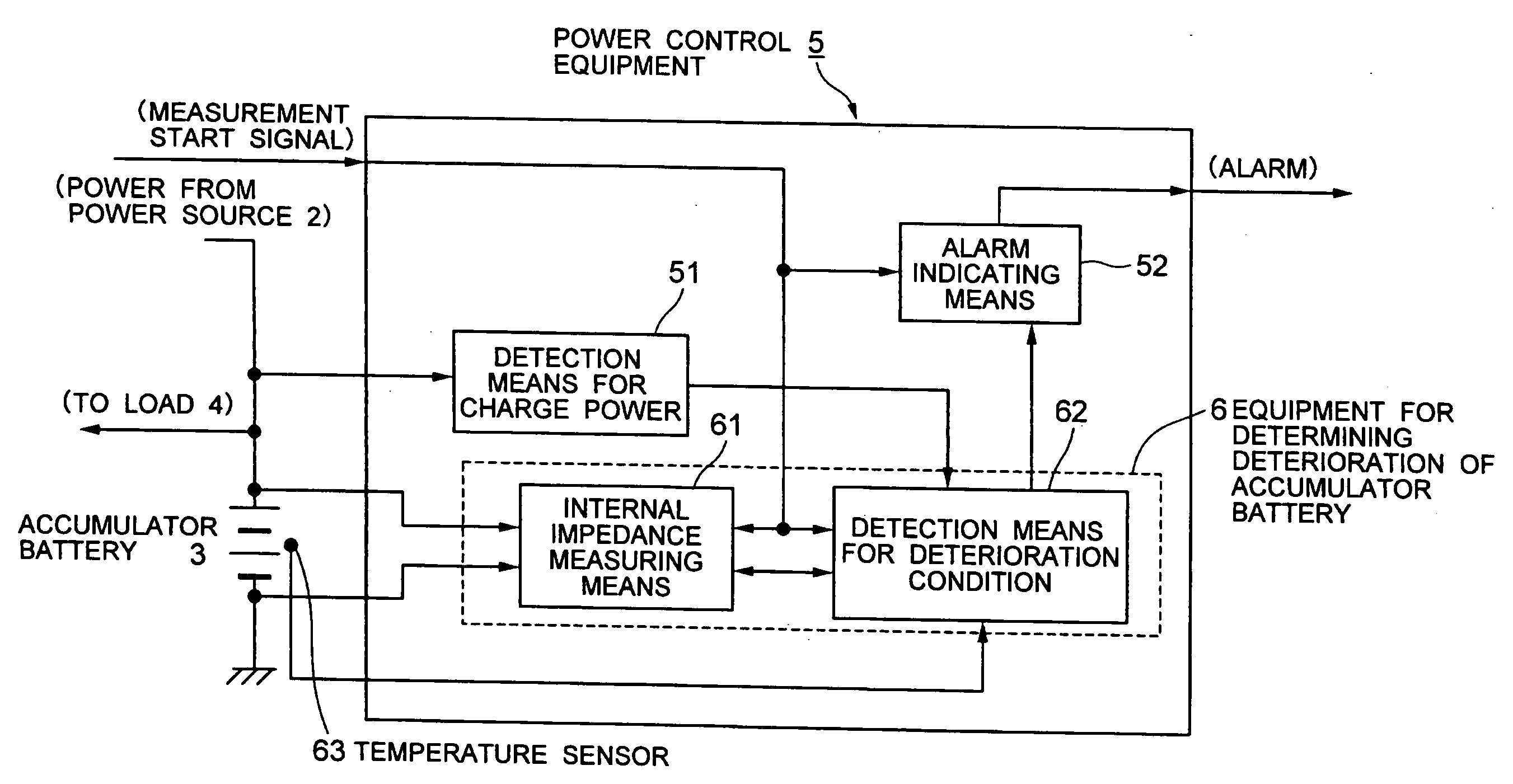
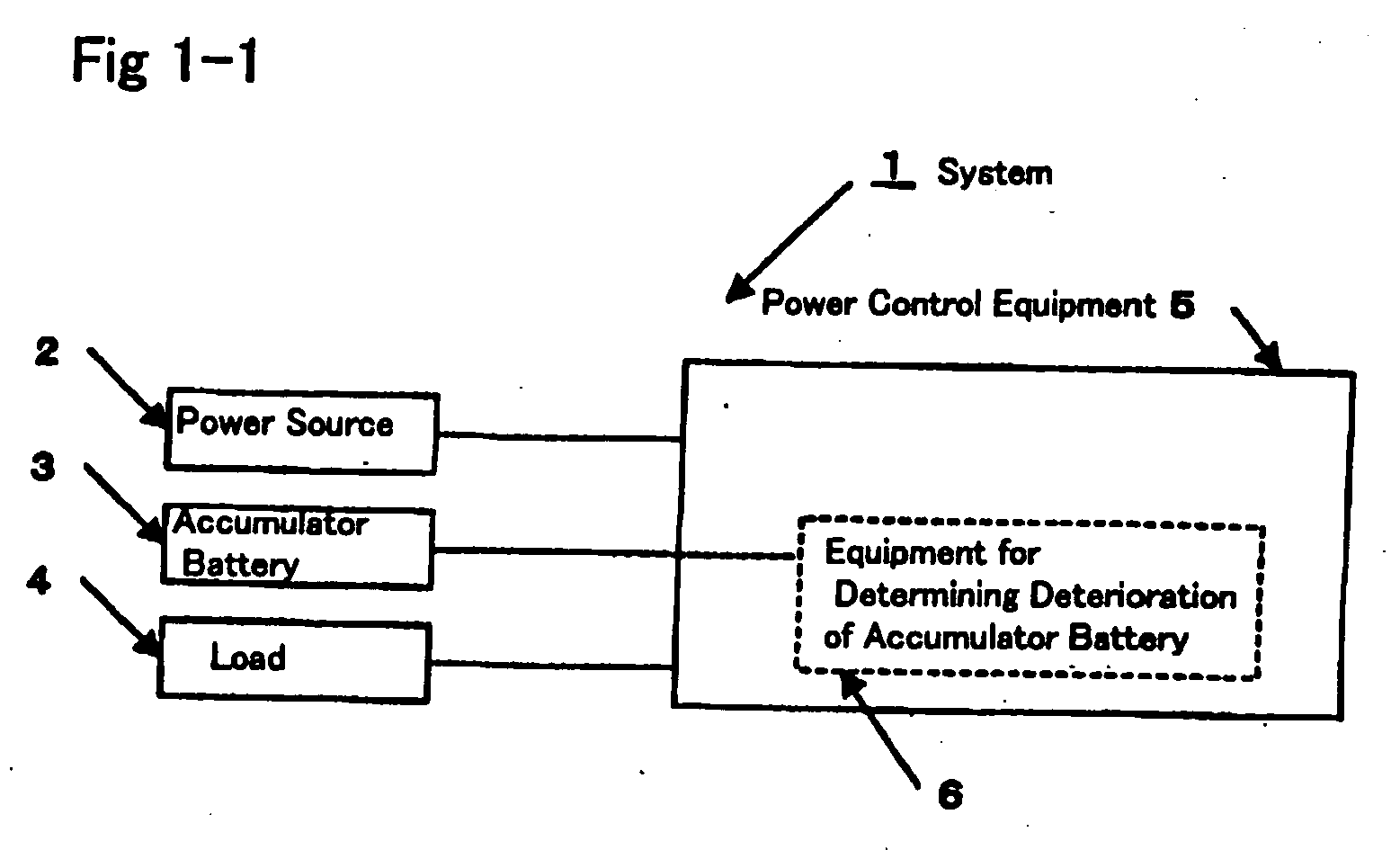
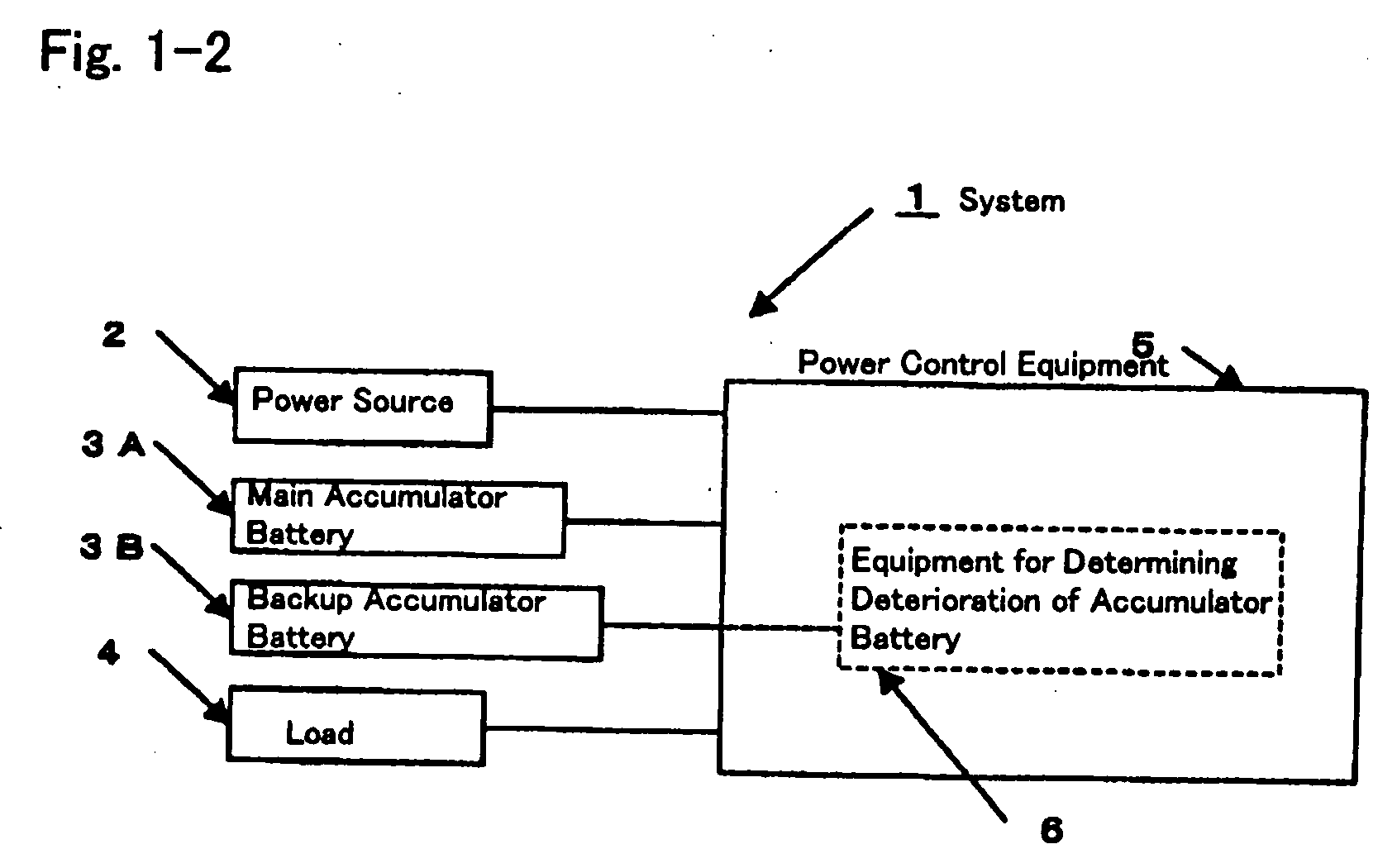
Method for determining deterioration of accumulator battery, method for measuring internal impedance of secondary battery, equipment for measuring internal impedance of secondary battery, equipment for determining deterioration of secondary battery, and power supply system
ActiveUS20060186890A1Complicated processIncrease equipment costBatteries circuit arrangementsMaterial analysis by electric/magnetic meansElectrical resistance and conductanceConversion factor
A method for determining deterioration of accumulator battery hooked up with loads in a system based on results of measuring internal resistances of an accumulator battery, the method comprising the steps of; predetermining as a specified temperature a temperature at which the deterioration of the accumulator battery is determined; calculating in advance temperature correction coefficients of the internal resistances from changes of the internal resistances depending on temperatures; predetermining resistance-voltage conversion factors to convert between the internal resistances at the specified temperature and terminal discharge voltages of the accumulator battery which are obtained at the specified temperature under a condition of flowing predetermined discharge currents from the accumulator battery; measuring the internal resistances of the accumulator battery and temperature of the accumulator battery at an internal resistance measurement; converting the measured internal resistance values into the internal resistance values at the specified temperature with use of the temperature correction coefficients of the internal resistances; converting the internal resistance values at the specified temperature into the terminal discharge voltage values of the accumulator battery at the specified temperature with use of the resistance-voltage conversion factors; and determining whether the accumulator battery is deteriorated or not by means of comparison of the terminal discharge voltage values of the accumulator battery at the specified temperature and a predetermined threshold value as a deterioration judgment standard.
Owner:FURUKAWA ELECTRIC CO LTD
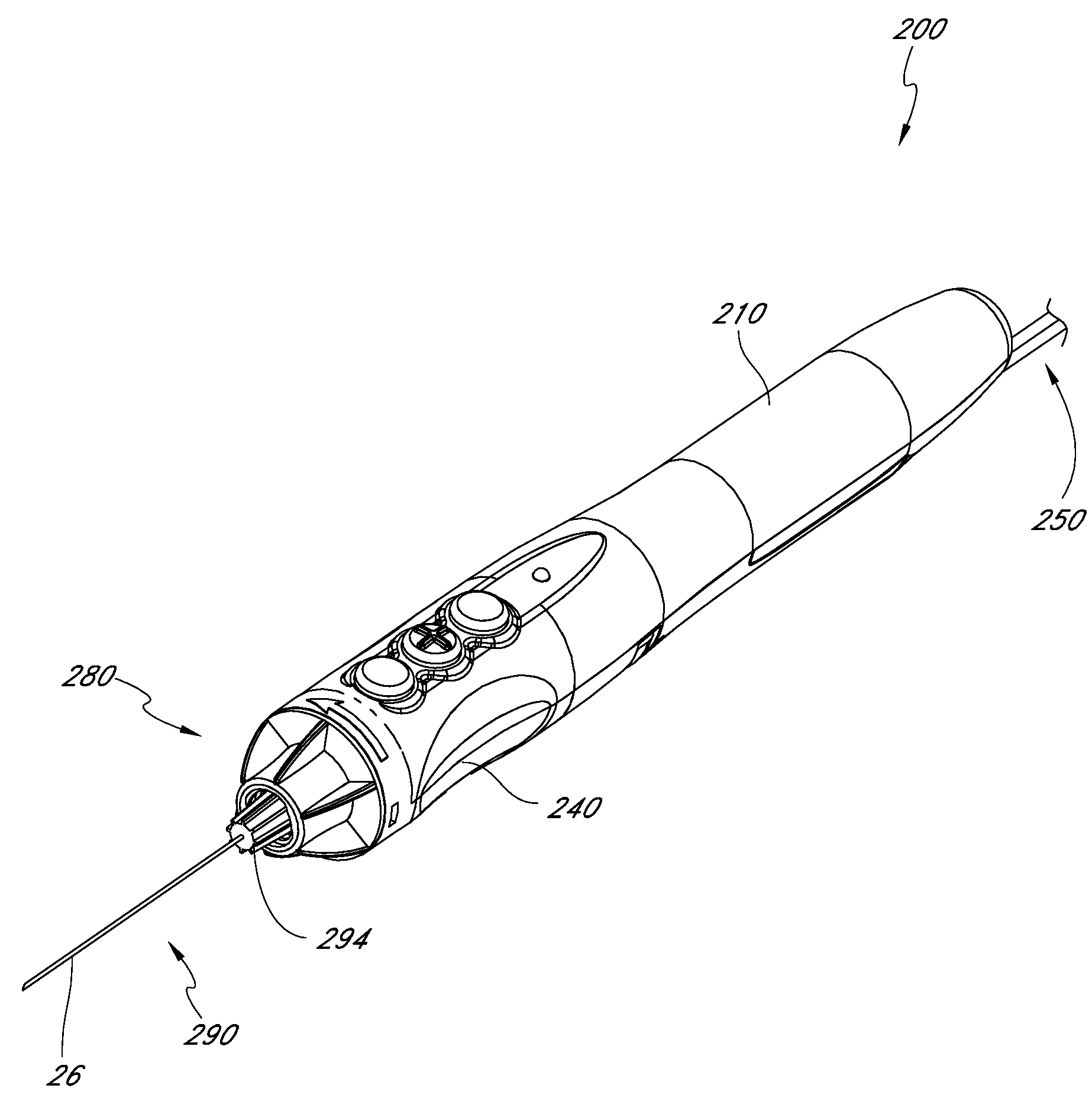
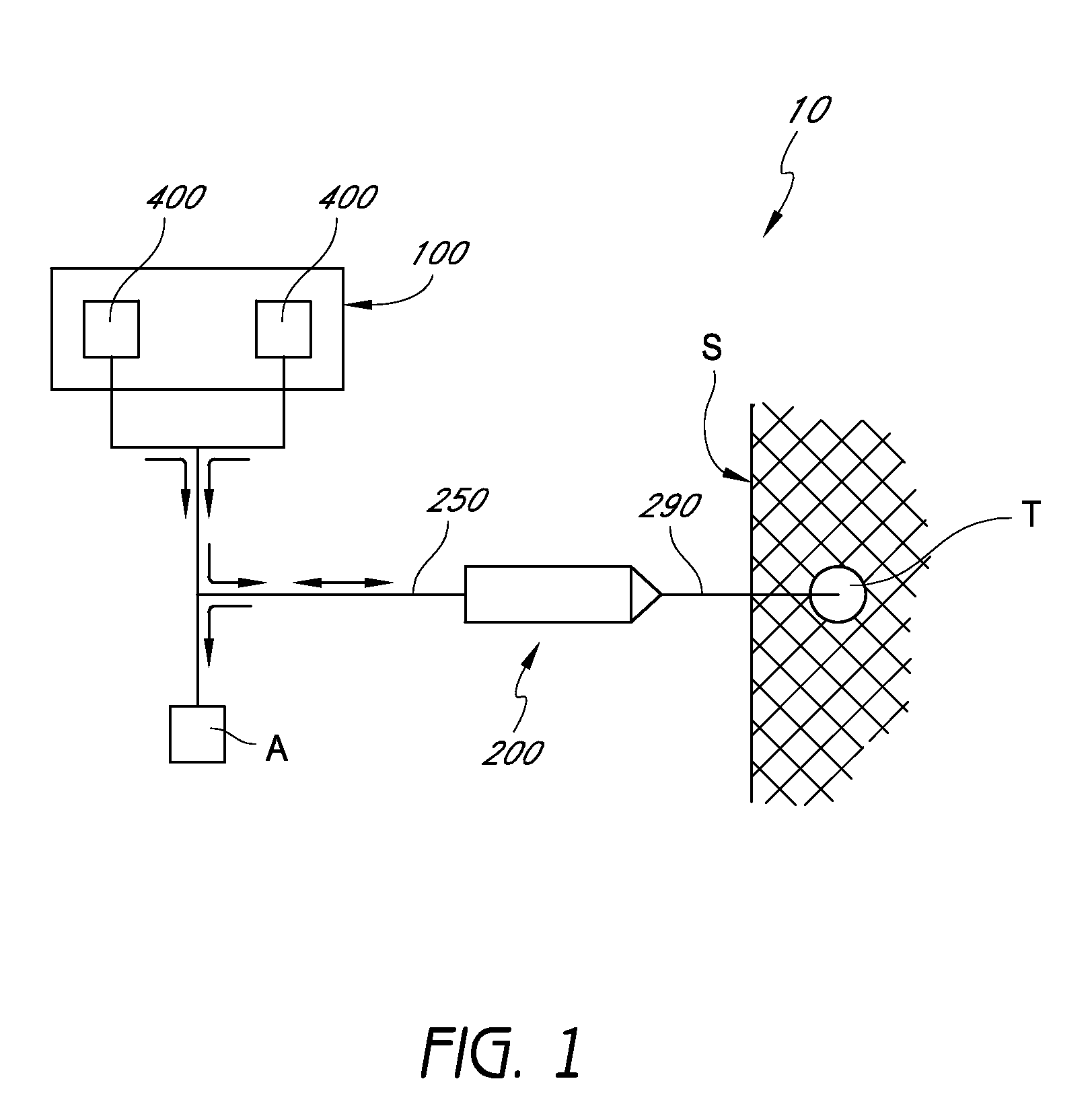
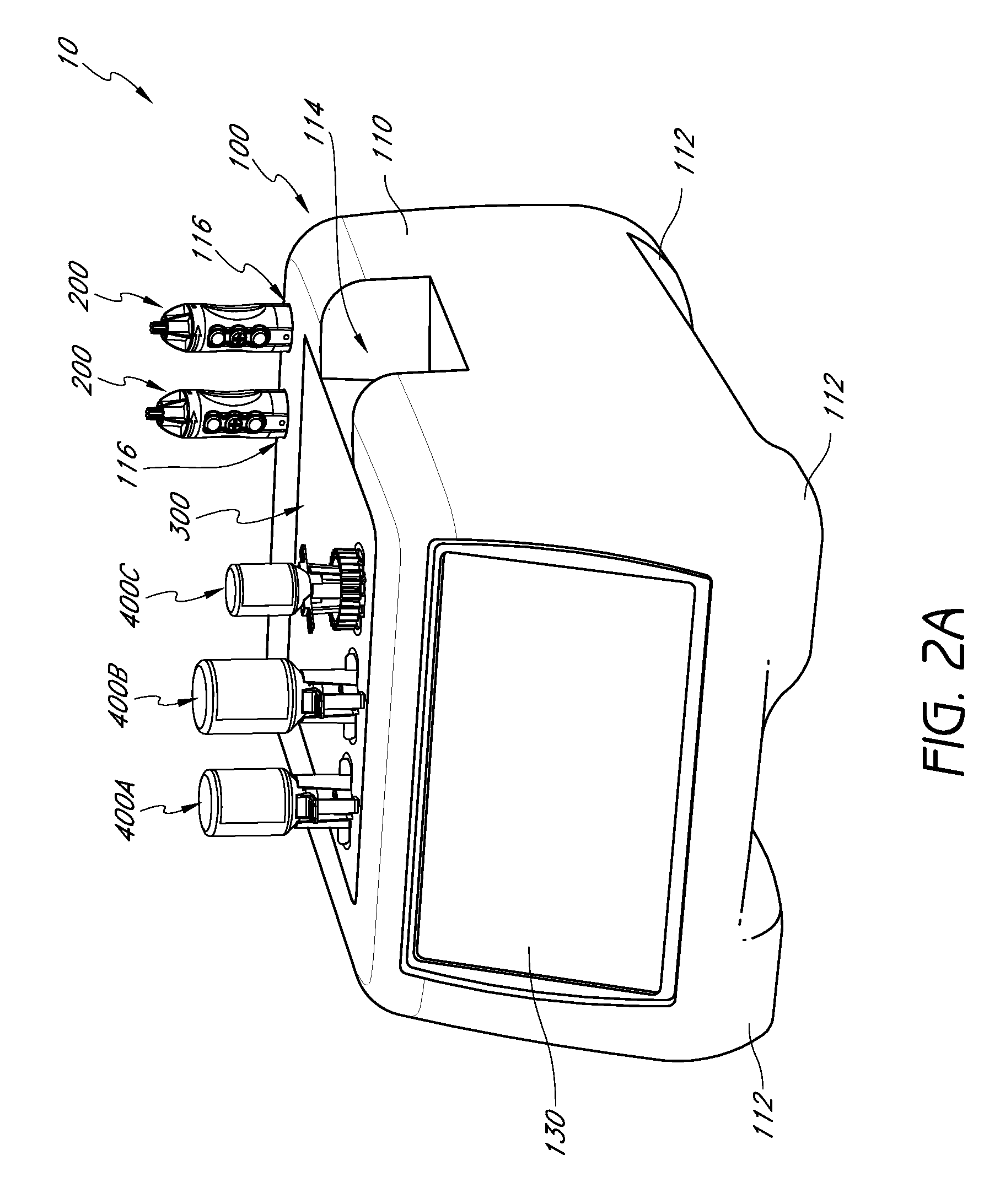
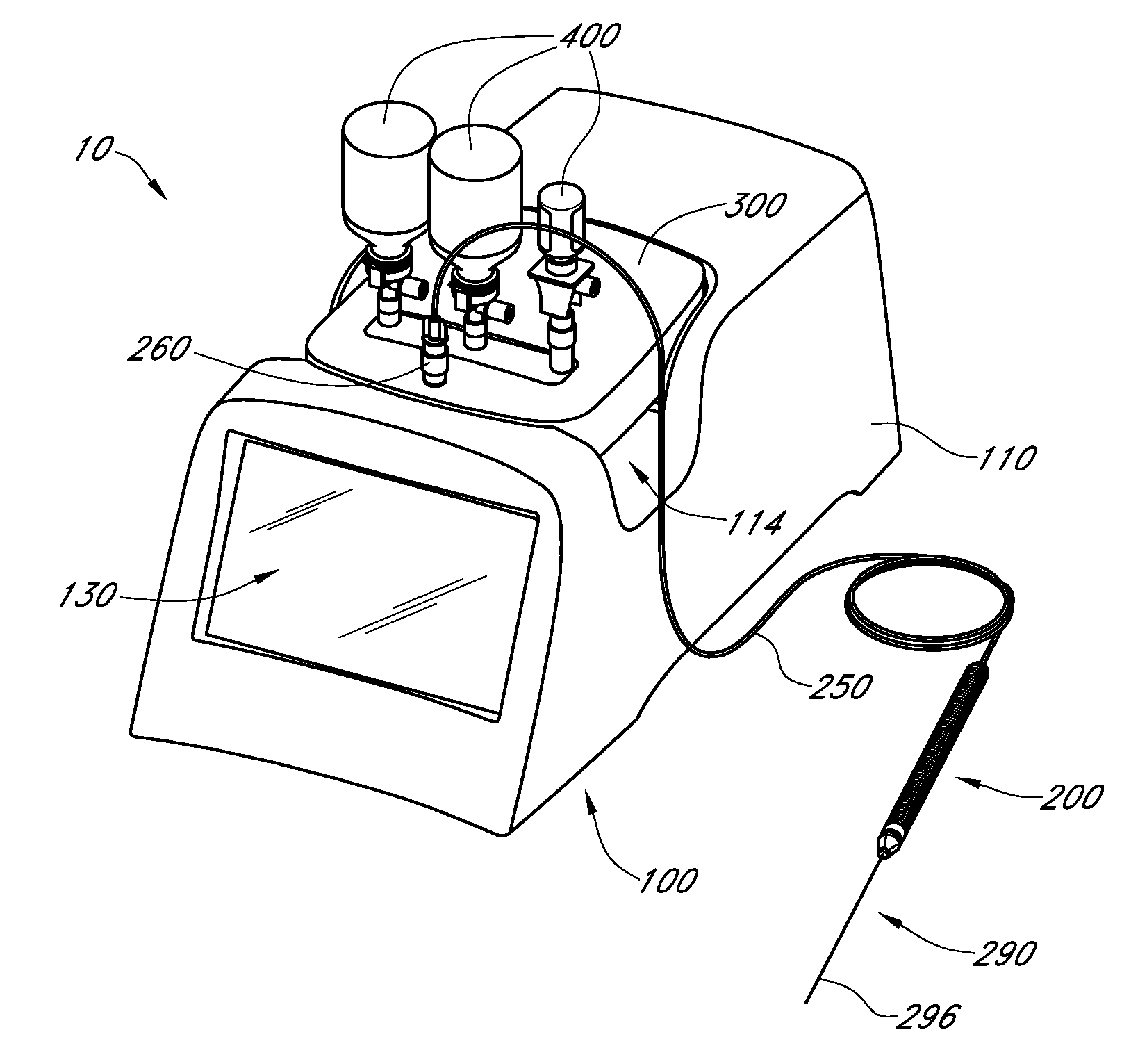
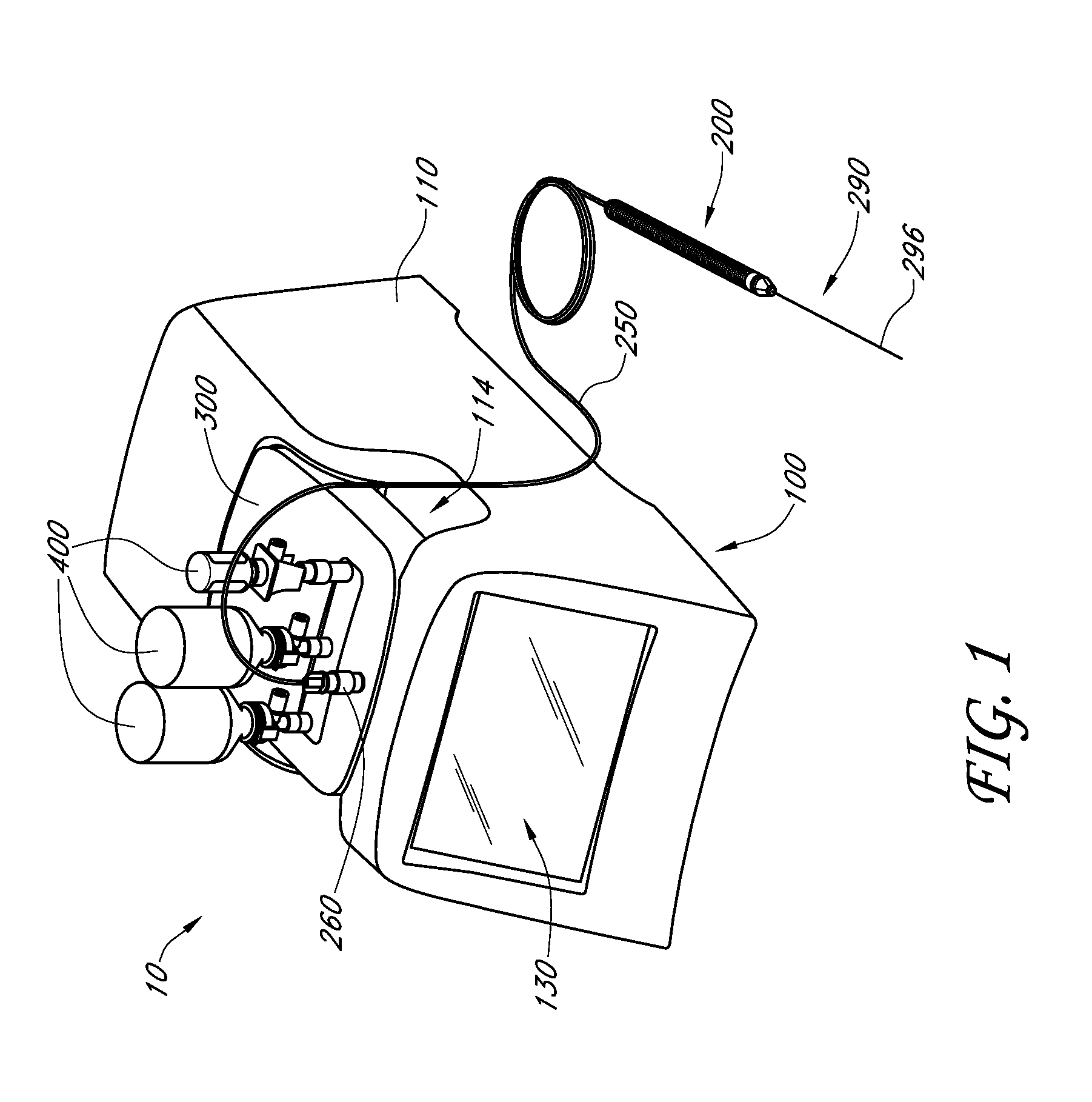
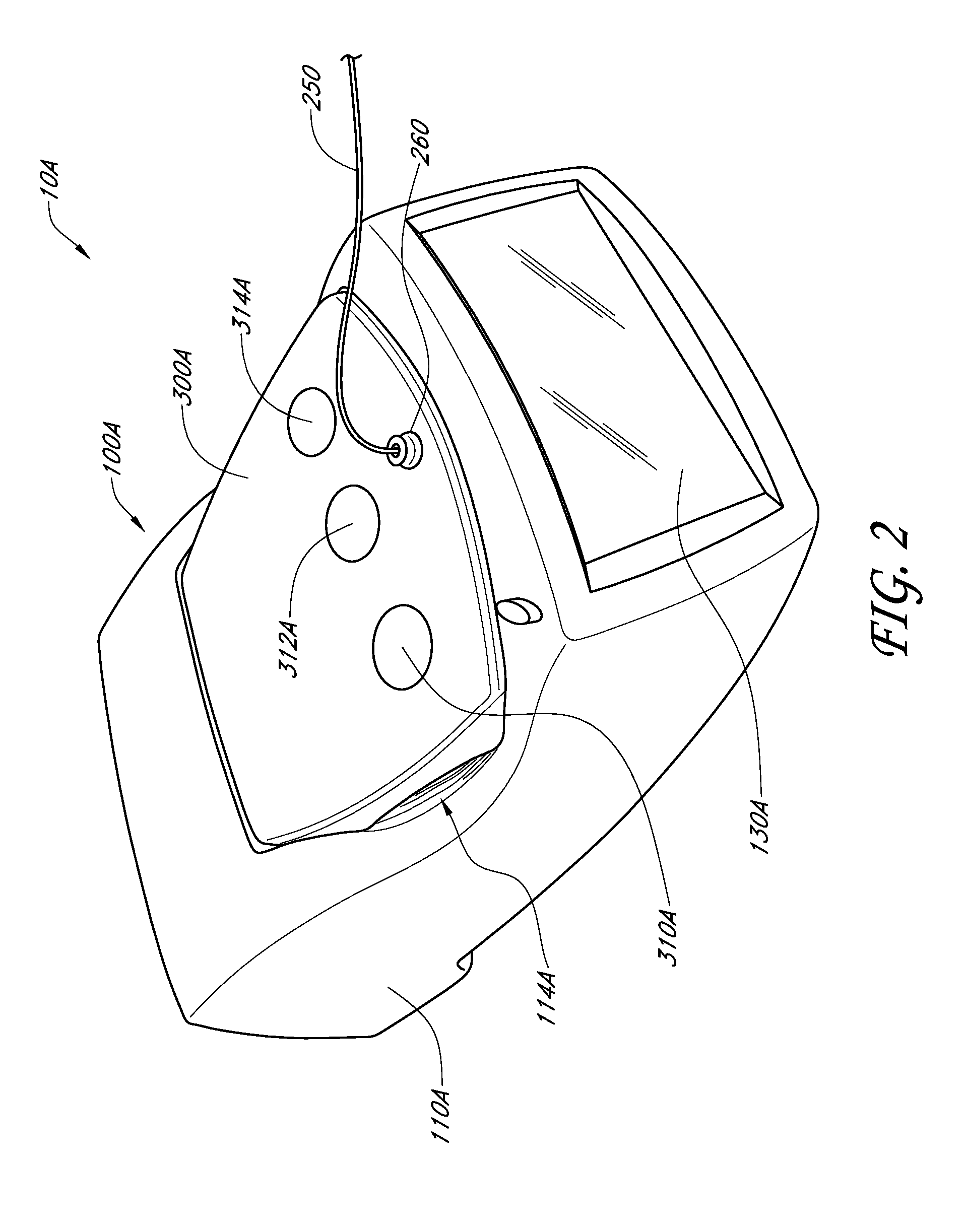
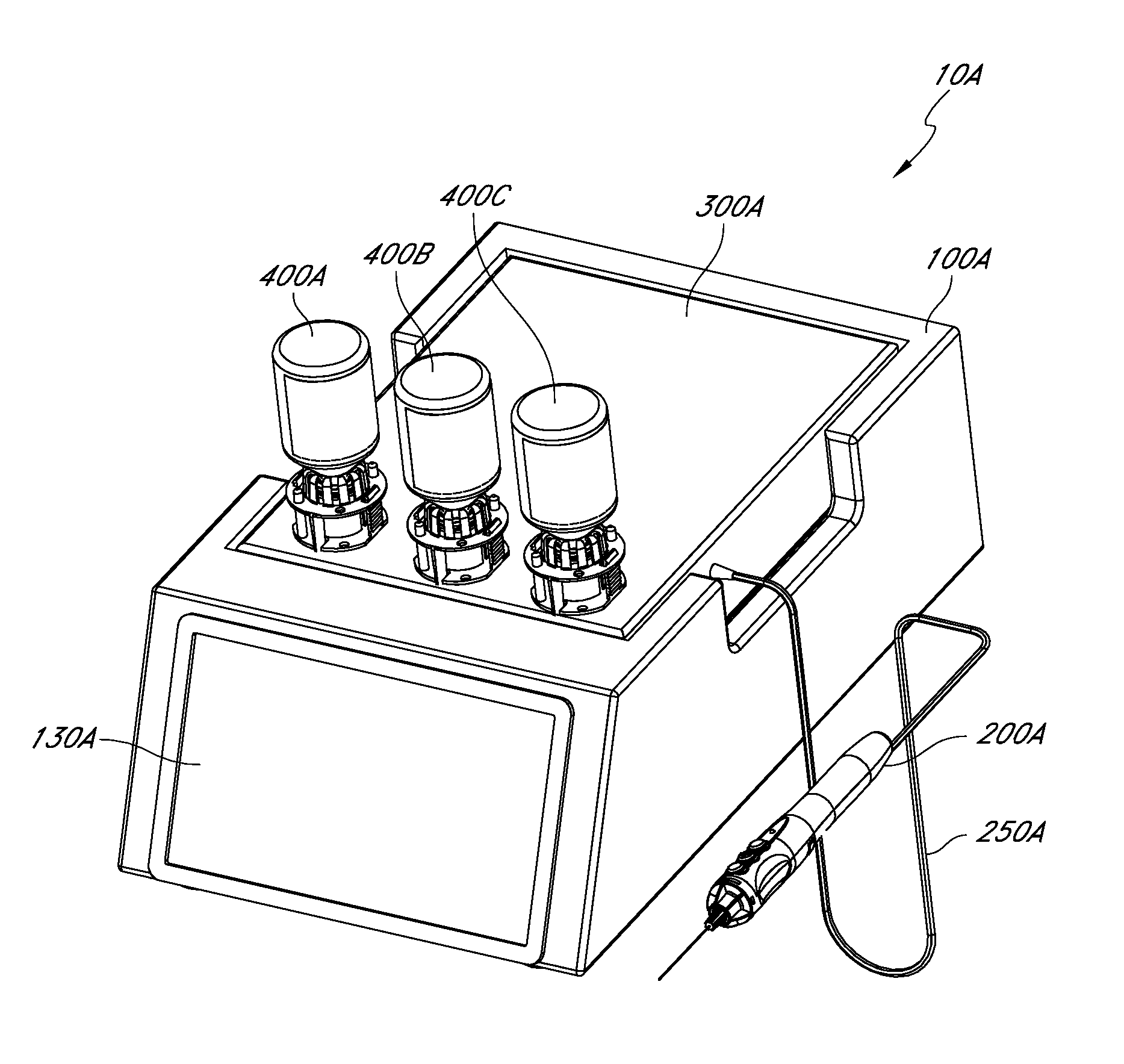
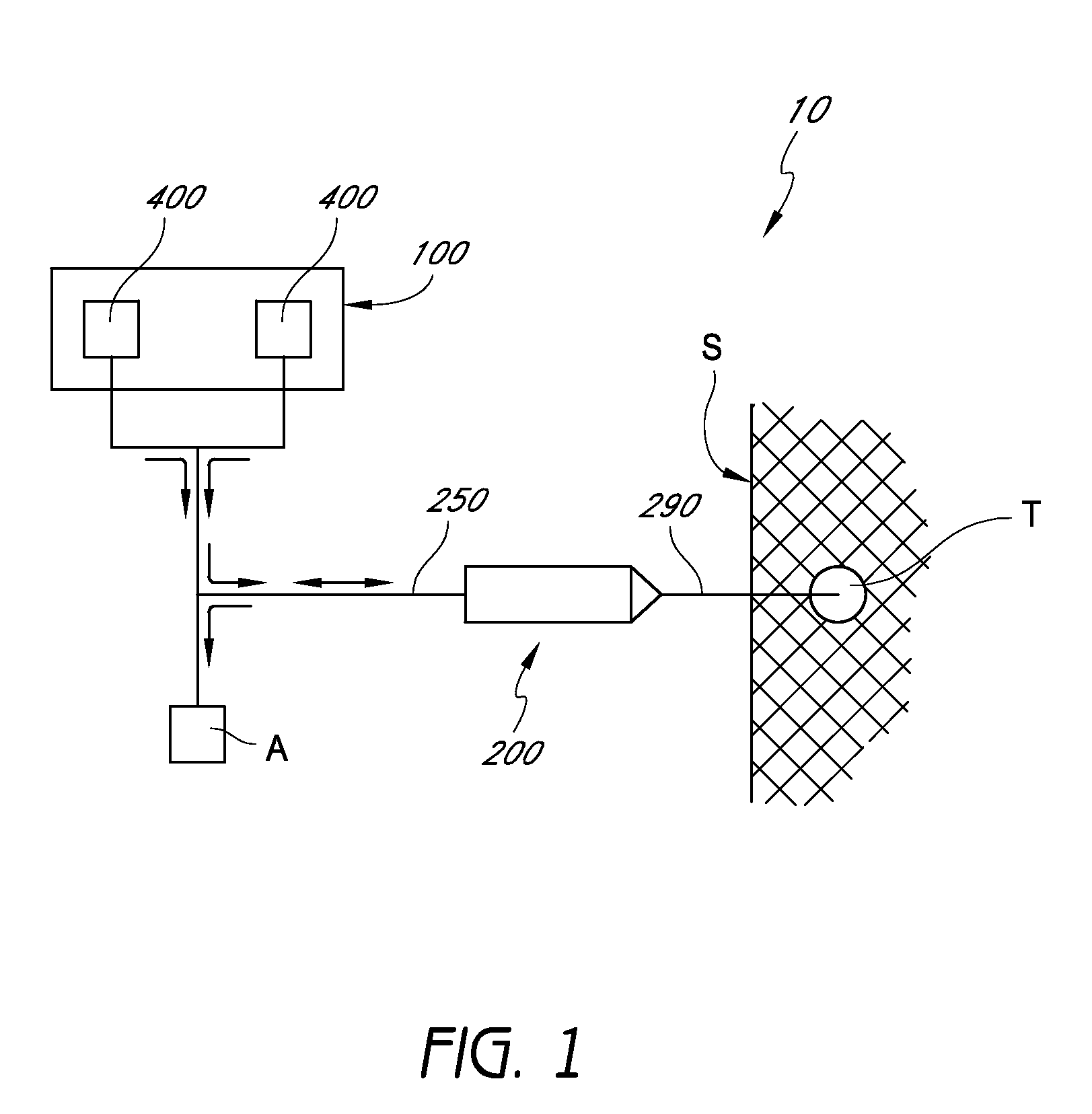
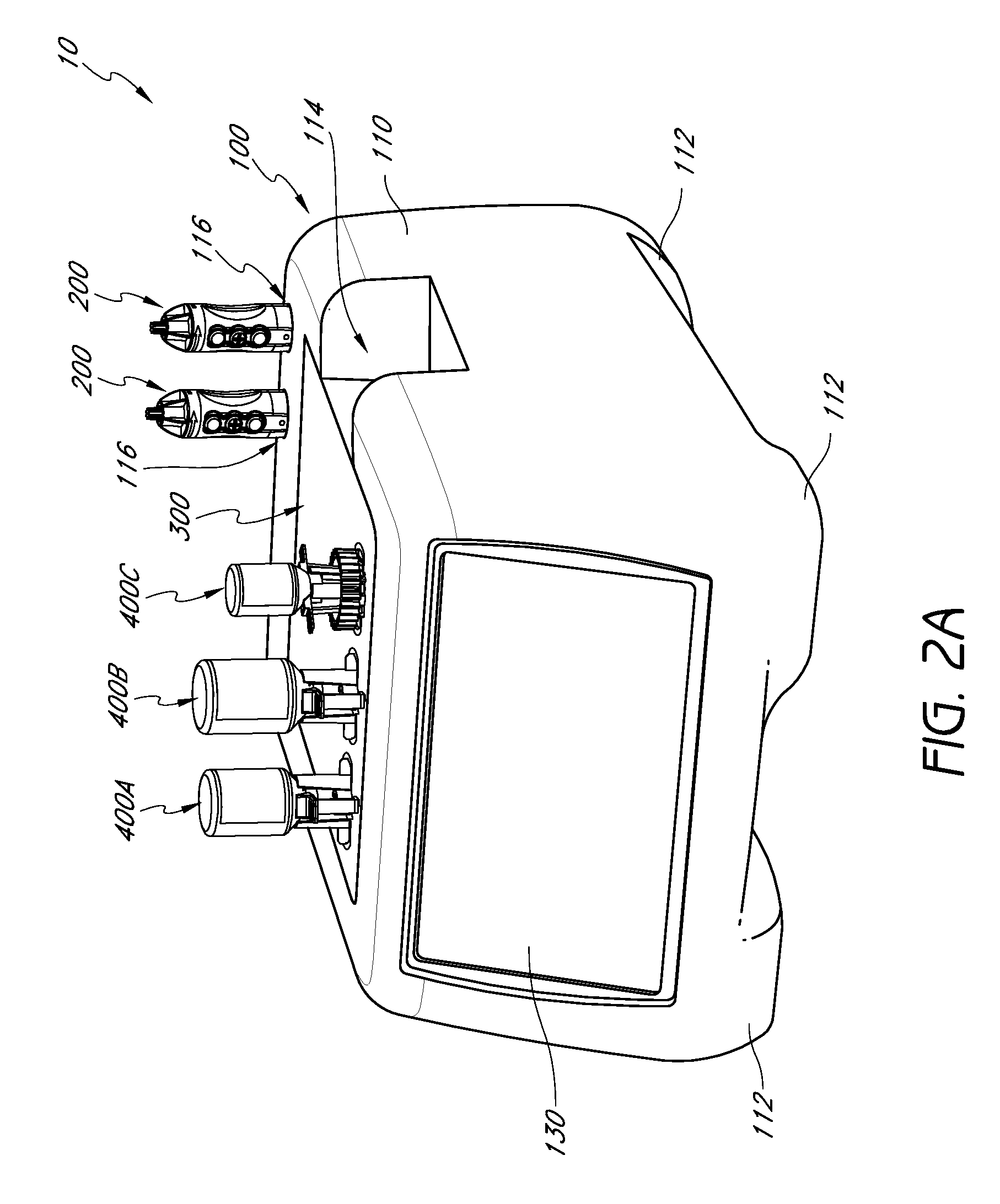
Handpiece assembly for articular injection systems
InactiveUS20090163860A1Precise deliveryPatient benefitUltrasonic/sonic/infrasonic diagnosticsDrug and medicationsJoint injectionSacroiliac joint
Systems for injecting fluids and / or other materials into a targeted anatomical location, in particular, an intra-articular space, include a handpiece assembly having a proximal end and a distal end, a needle extending from the distal end of the handpiece assembly, a fluid delivery module comprising a cassette and a fluid transfer device. A conduit is generally configured to place the fluid delivery module in fluid communication with the handpiece assembly. Medications, formulations and / or other fluids or materials contained within vials that are secured to the fluid delivery module can be selectively delivered into an anatomy through a needle located at the distal end of the handpiece assembly. In some embodiments, ultrasound or other imaging technologies can be used to locate a joint or other targeted anatomical location.
Owner:CARTICEPT MEDICAL
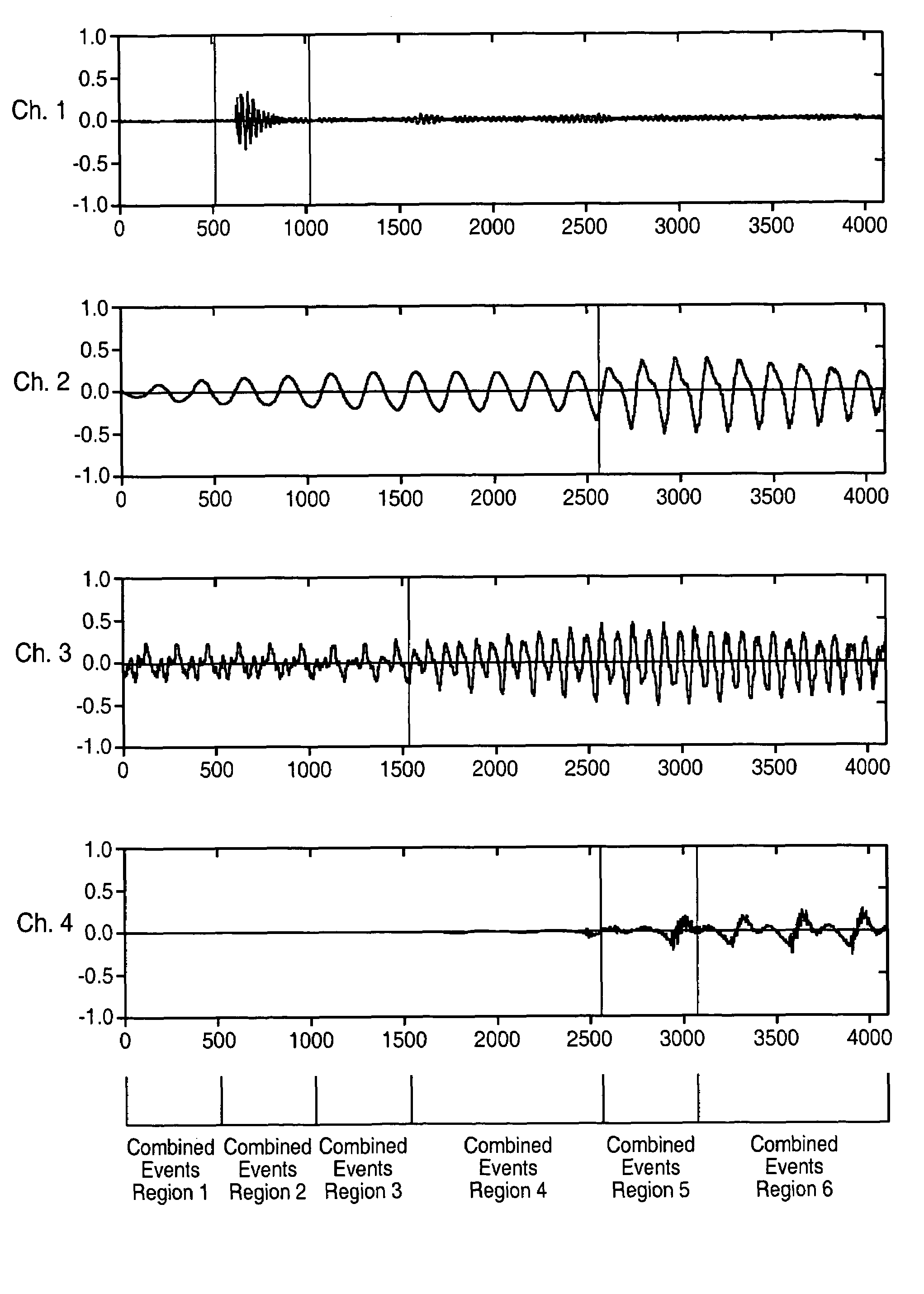
Segmenting audio signals into auditory events
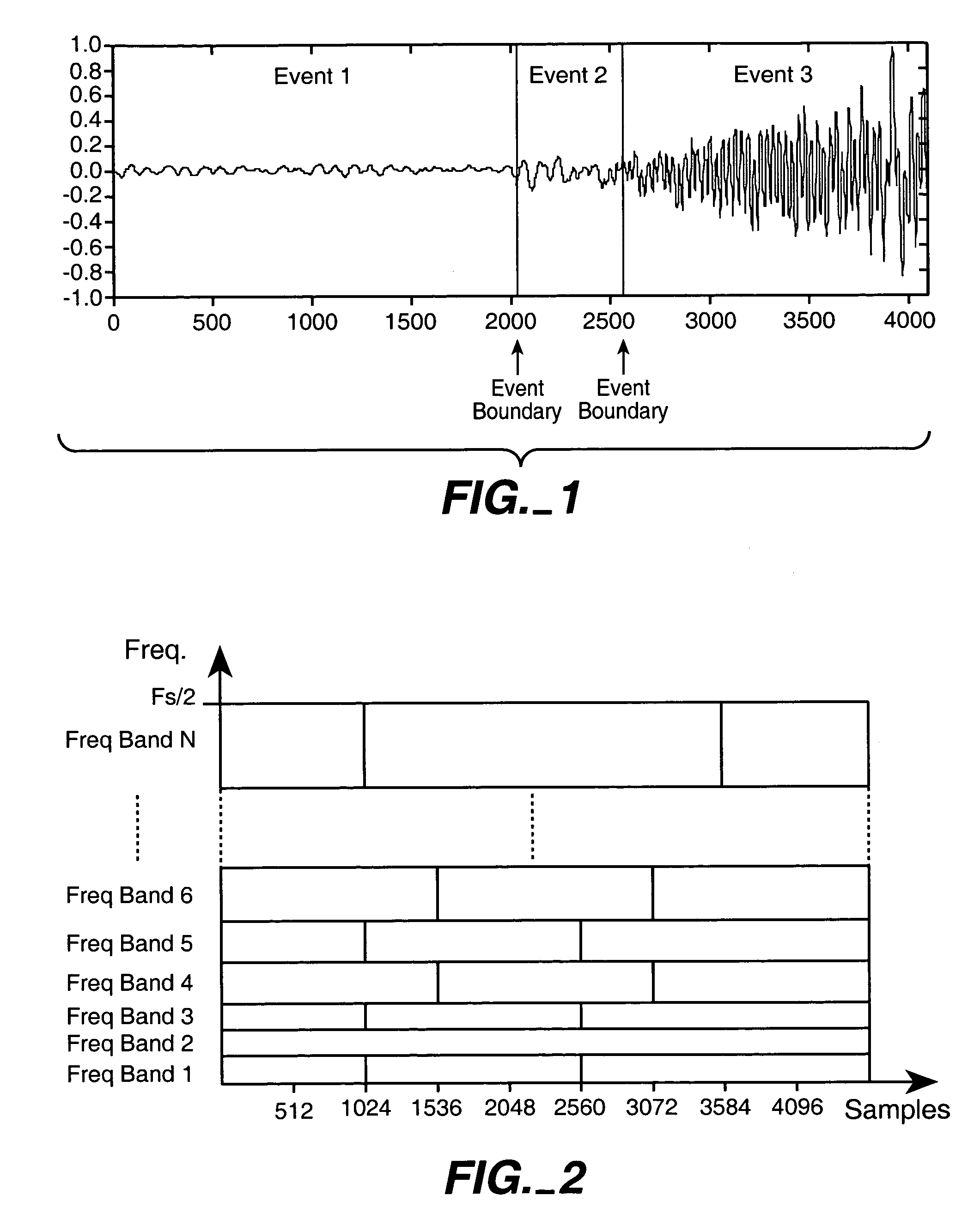
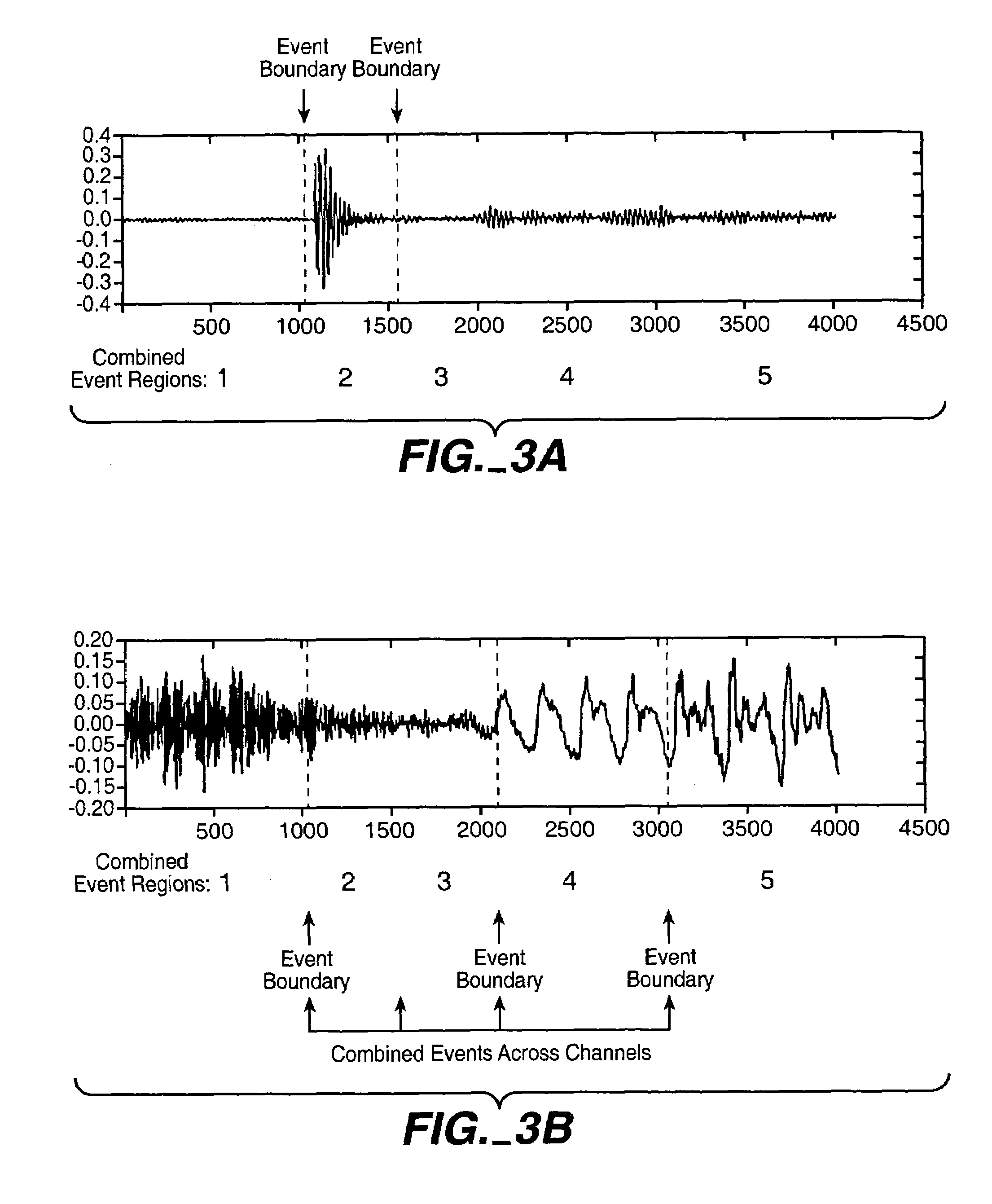
InactiveUS7711123B2Valuable informationGreat weightTelevision system detailsColor television detailsFrequency spectrumInformation representation
In one aspect, the invention divides an audio signal into auditory events, each of which tends to be perceived as separate and distinct, by calculating the spectral content of successive time blocks of the audio signal (5-1), calculating the difference in spectral content between successive time blocks of the audio signal (5-2), and identifying an auditory event boundary as the boundary between successive time blocks when the difference in the spectral content between such successive time blocks exceeds a threshold (5-3). In another aspect, the invention generates a reduced-information representation of an audio signal by dividing an audio signal into auditory events, each of which tends to be perceived as separate and distinct, and formatting and storing information relating to the auditory events (5-4). Optionally, the invention may also assign a characteristic to one or more of the auditory events (5-5).
Owner:DOLBY LAB LICENSING CORP
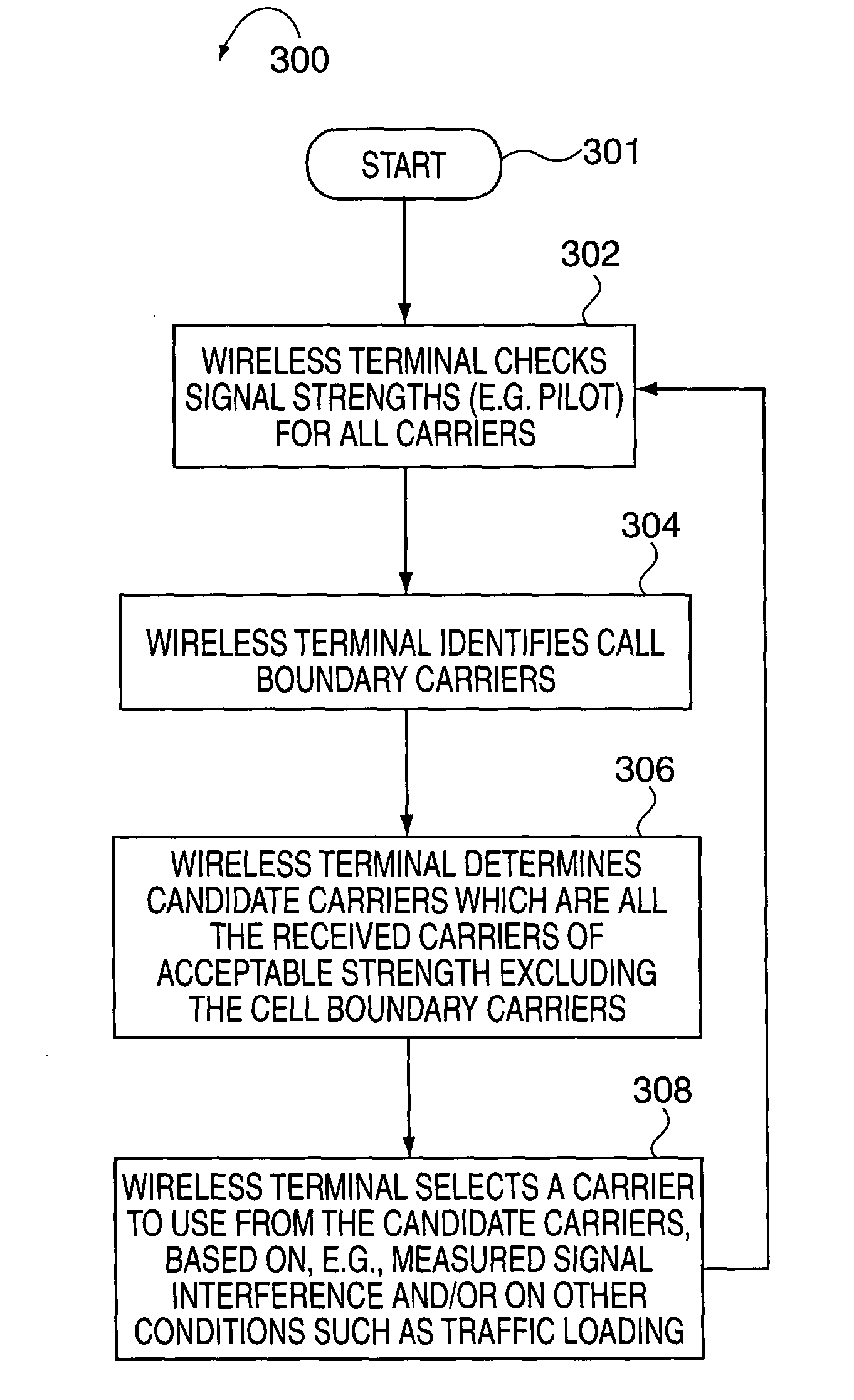
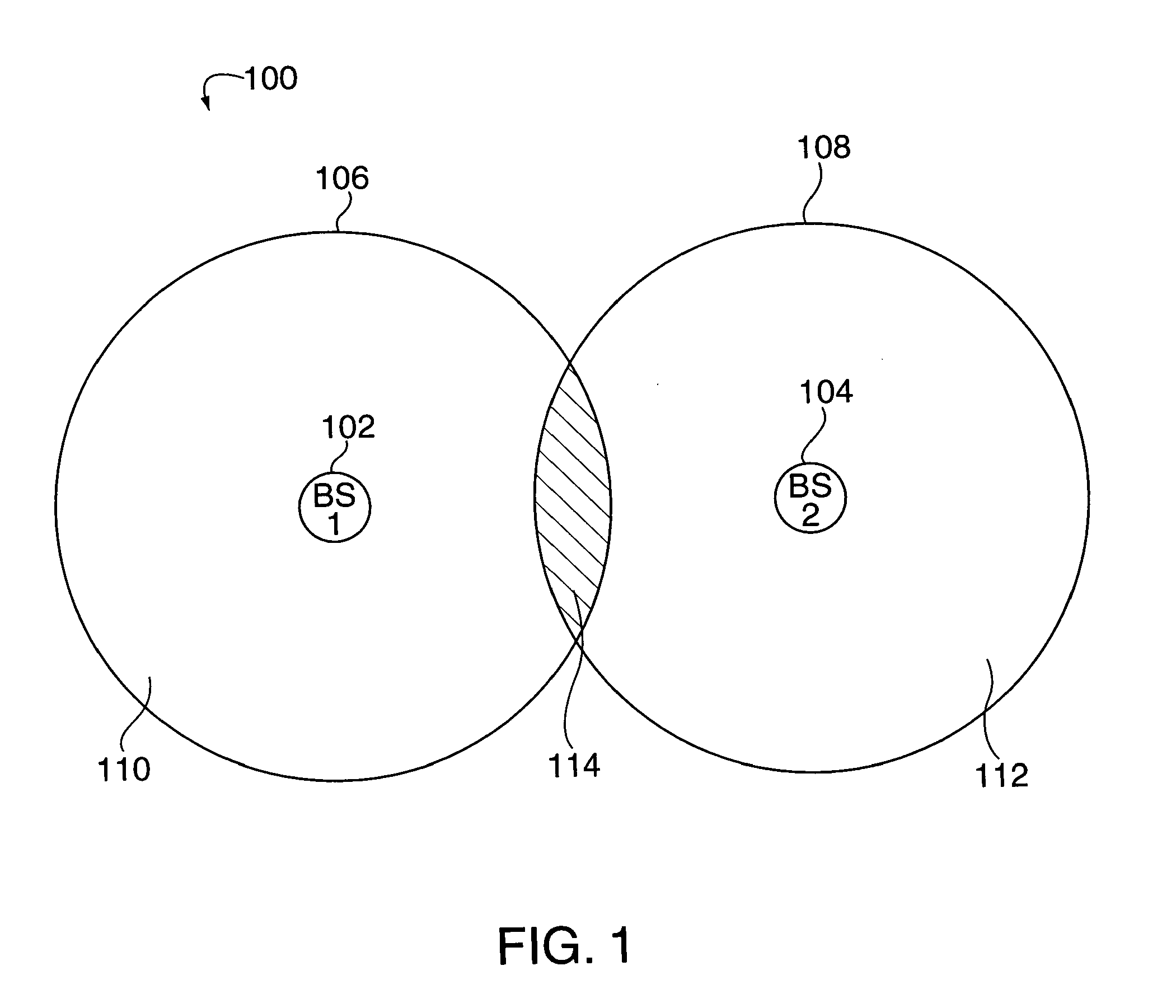
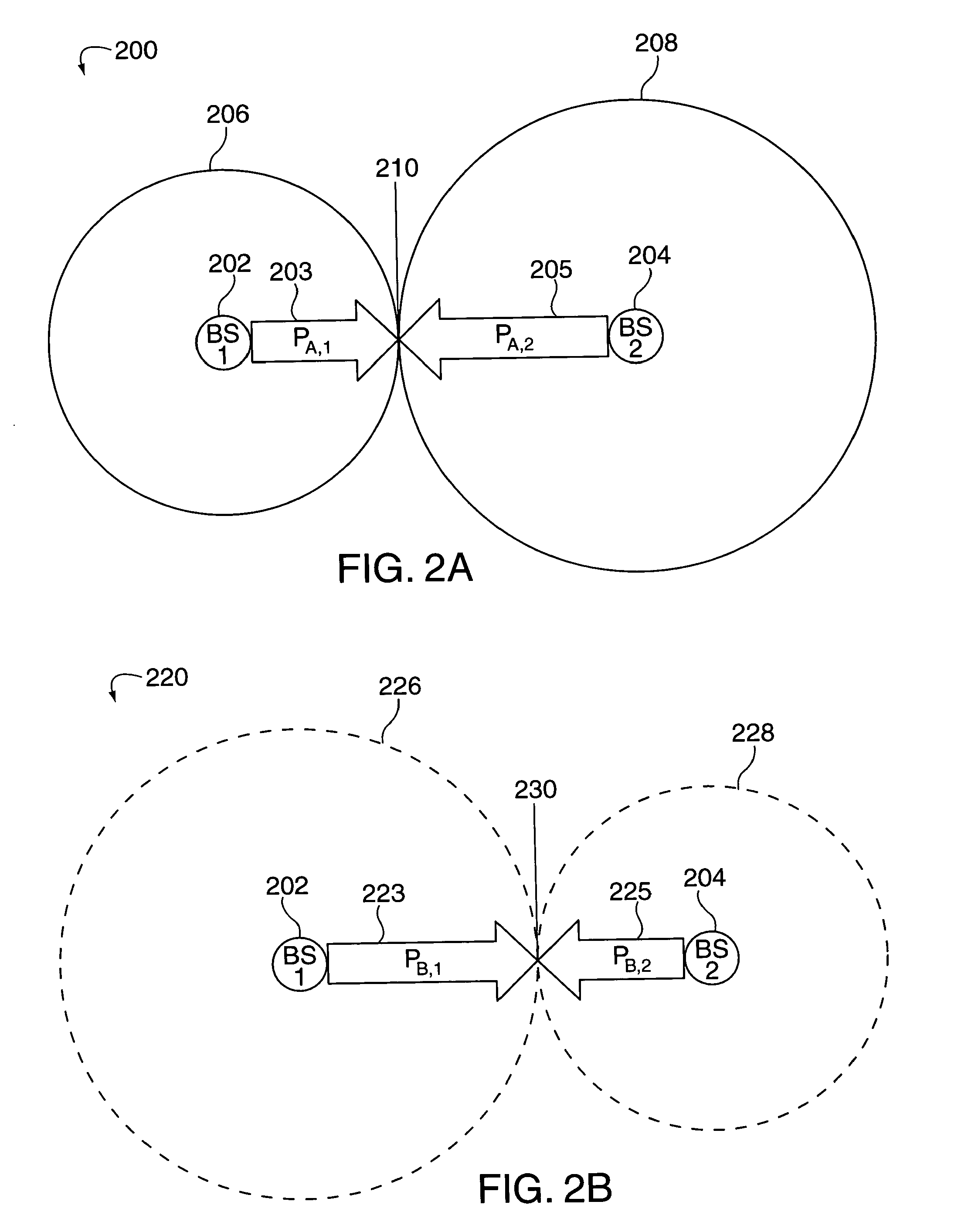
Method of creating and utilizing diversity in multiple carrier communication system
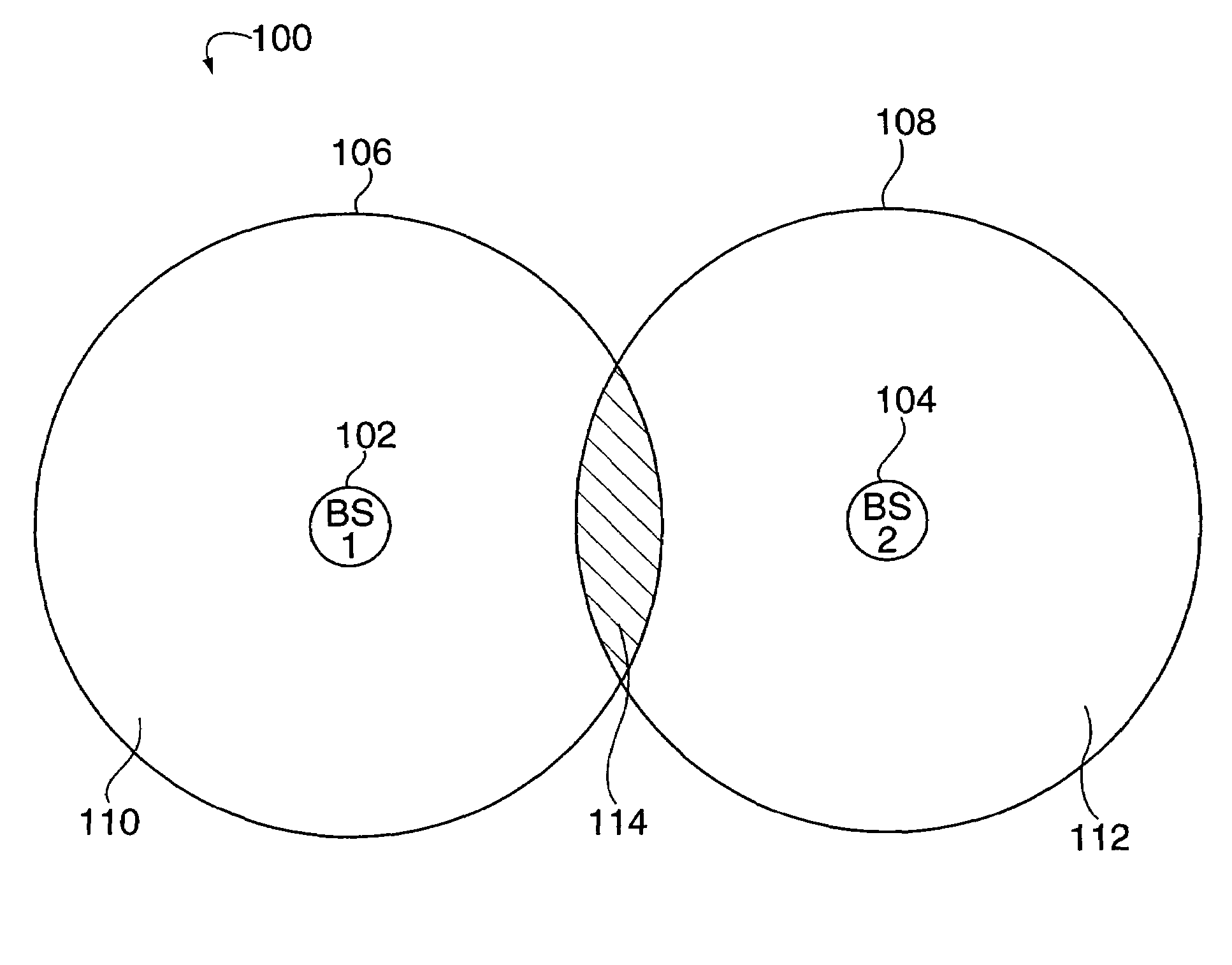
InactiveUS7363039B2Low reliabilityReduce quality problemsEnergy efficient ICTPower managementFrequency spectrumCommunications system
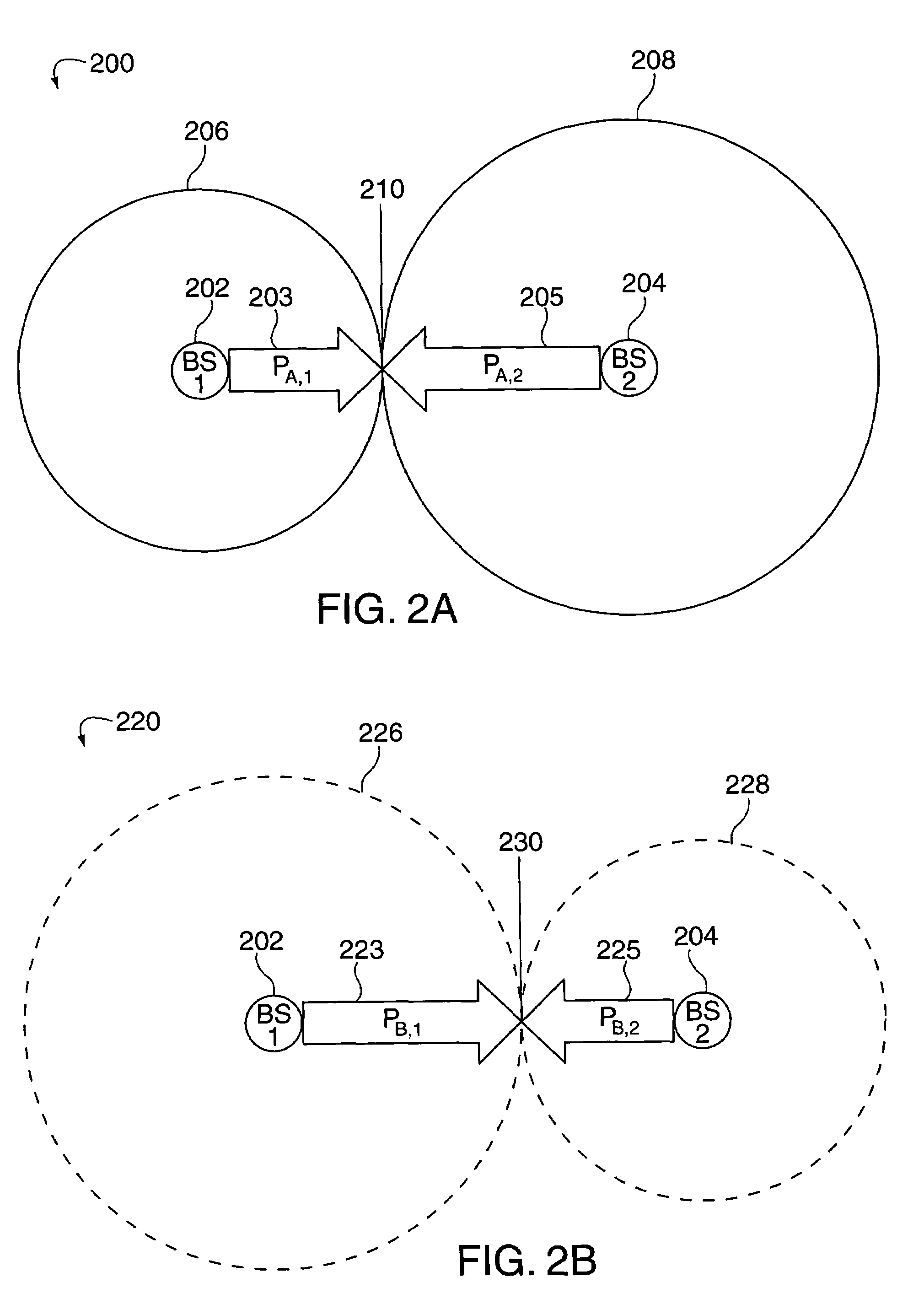
In many cellular systems, reusing spectrum bandwidth, creates problems in boundary regions between the cells and sectors where the signal strength received from adjacent base stations or adjacent sector transmissions of a single base station may be nearly equivalent. The invention creates a new type of diversity, referred to as multiple carrier diversity by utilizing multiple carriers, assigning different power levels to each carrier frequency at each base station, and / or offsetting sector antennas. The cell and / or sector coverage areas can be set so as to minimize or eliminate overlap between cell and / or sector boundary regions of different carrier frequencies. Mobile nodes traveling throughout the system can exploit multiple carrier diversity by detecting carriers and selecting to use a non-boundary carrier based on other system criteria in order to improve performance. Boundary carriers may, but need not be, identified and excluded from consideration for use by a wireless terminal.
Owner:QUALCOMM INC
Image display device
InactiveUS20090167658A1Effective luminance controlComplicated processStatic indicating devicesDisplay deviceLightness
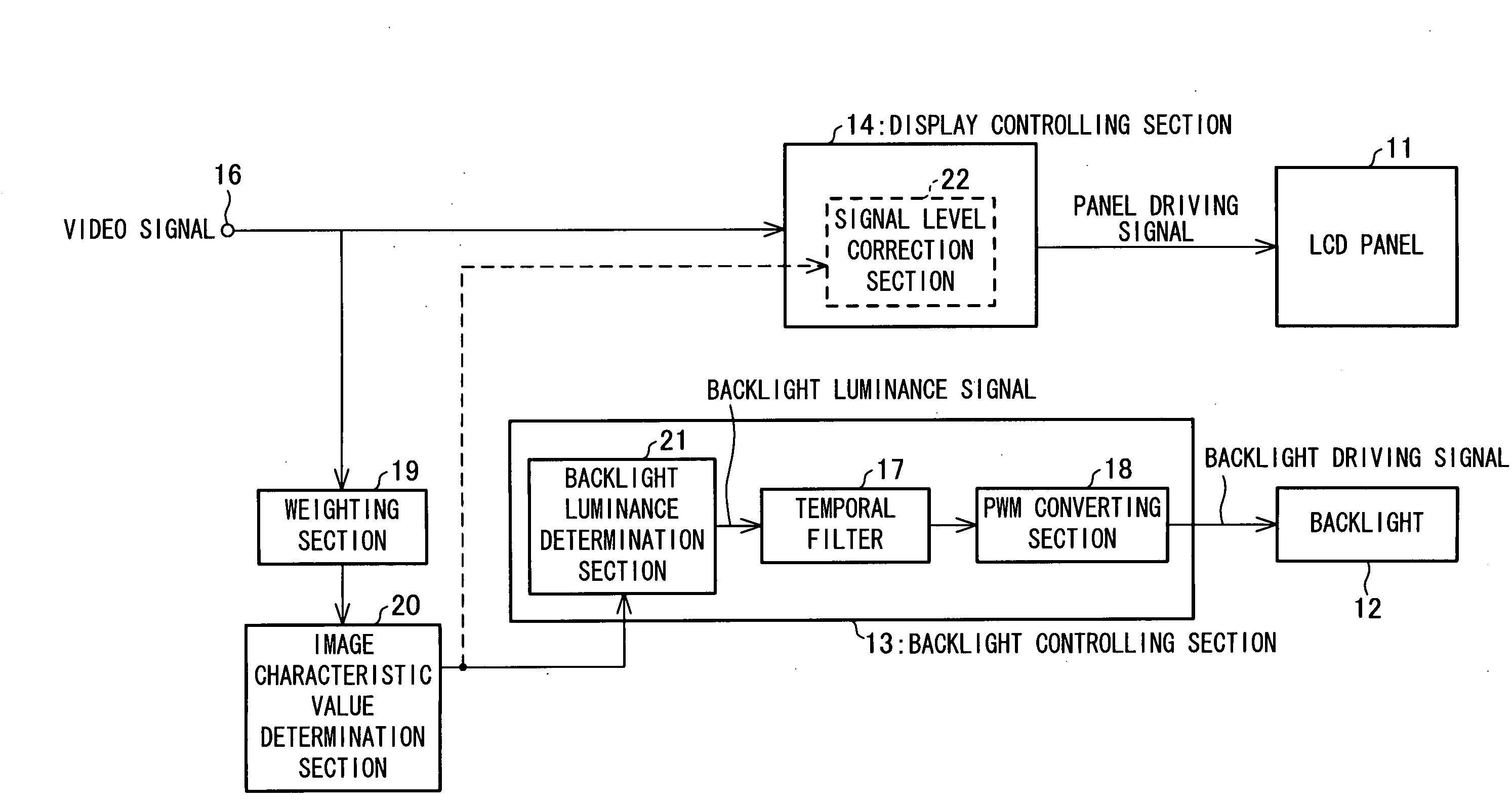
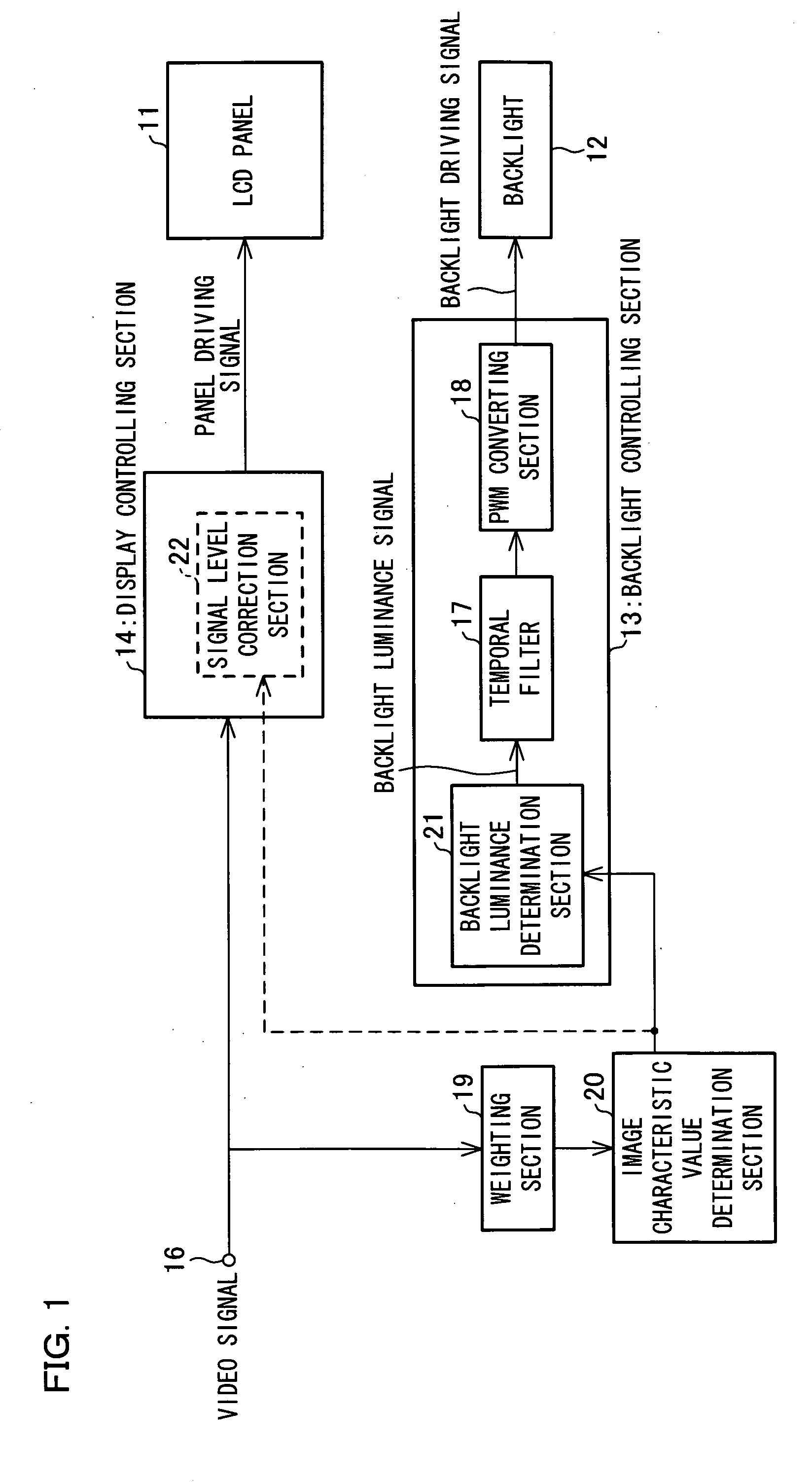
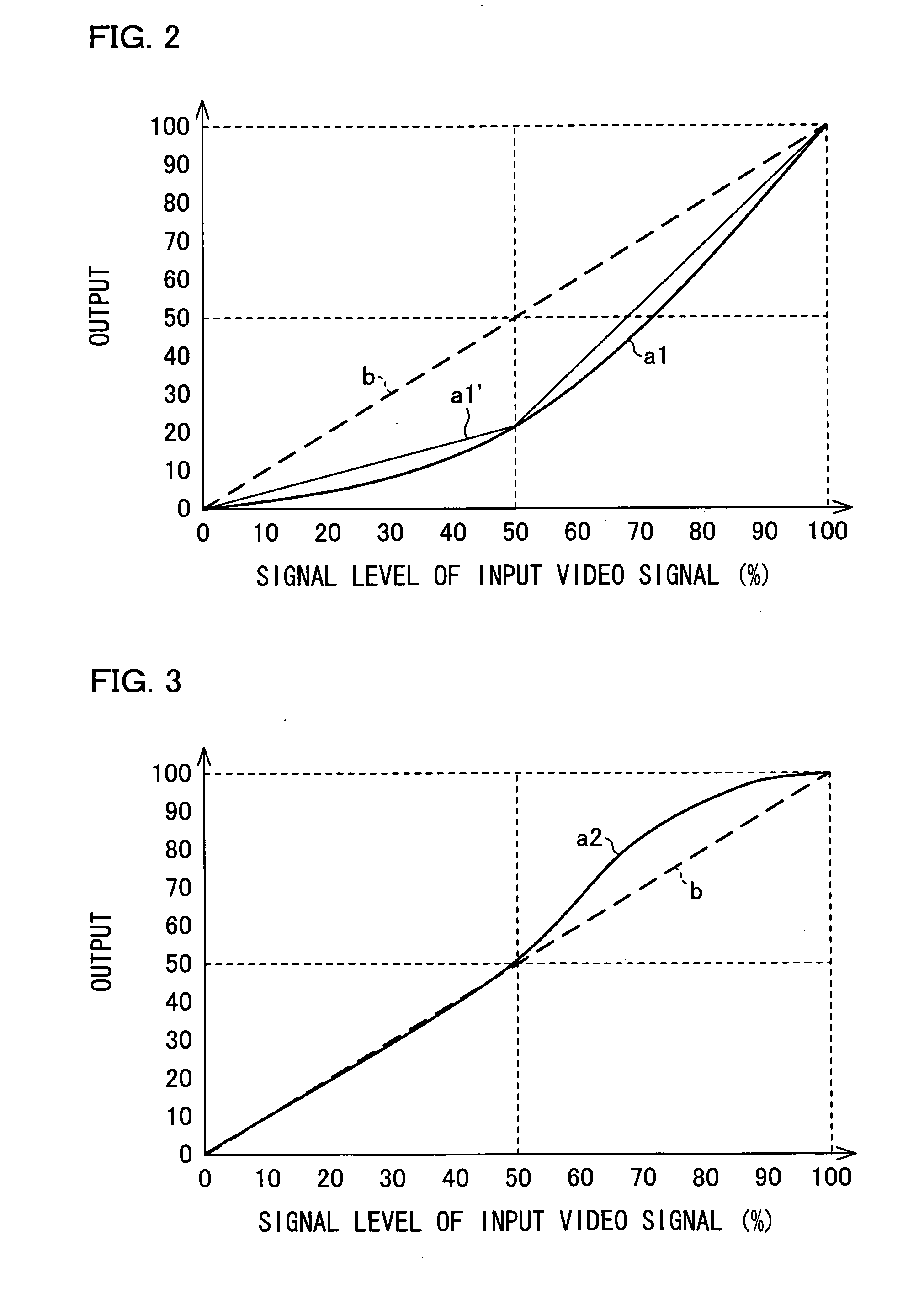
In one embodiment of the present invention, luminance information of a video signal inputted thereto is weighted by a weighting section. An image characteristic value determination section, by one frame, takes an average of the weighted luminance information, thereby determining an image characteristic value of one frame. A backlight controlling section sets luminance of the backlight at a backlight luminance determination section in accordance with the image characteristic value determined by the image characteristic value determination section and performs luminance correction.
Owner:SHARP KK
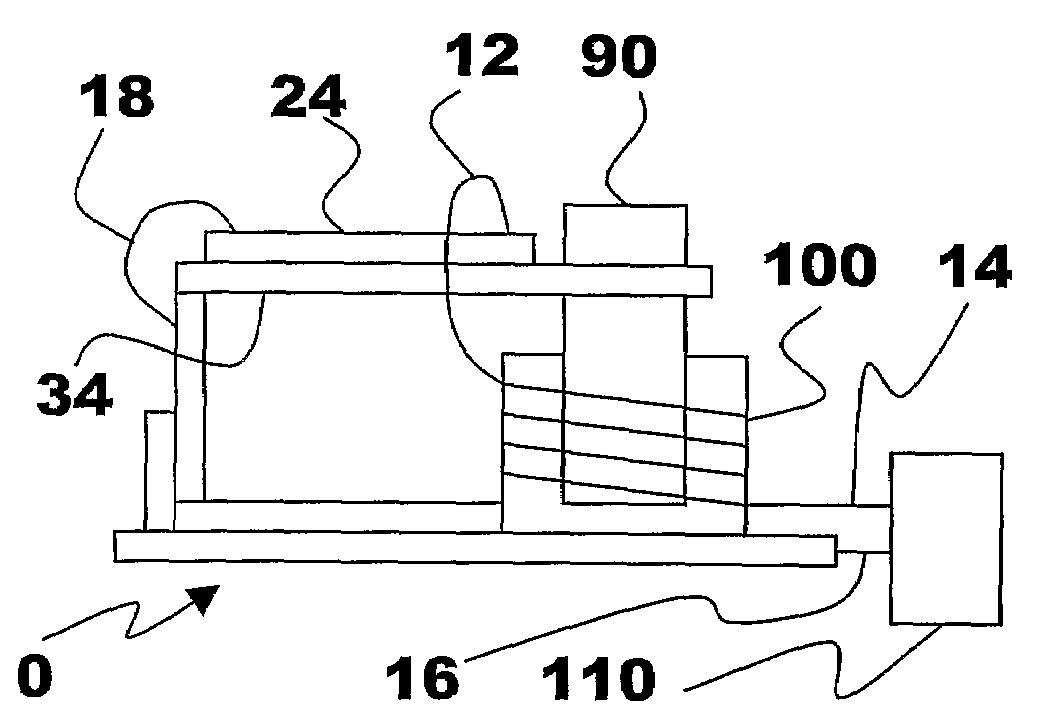
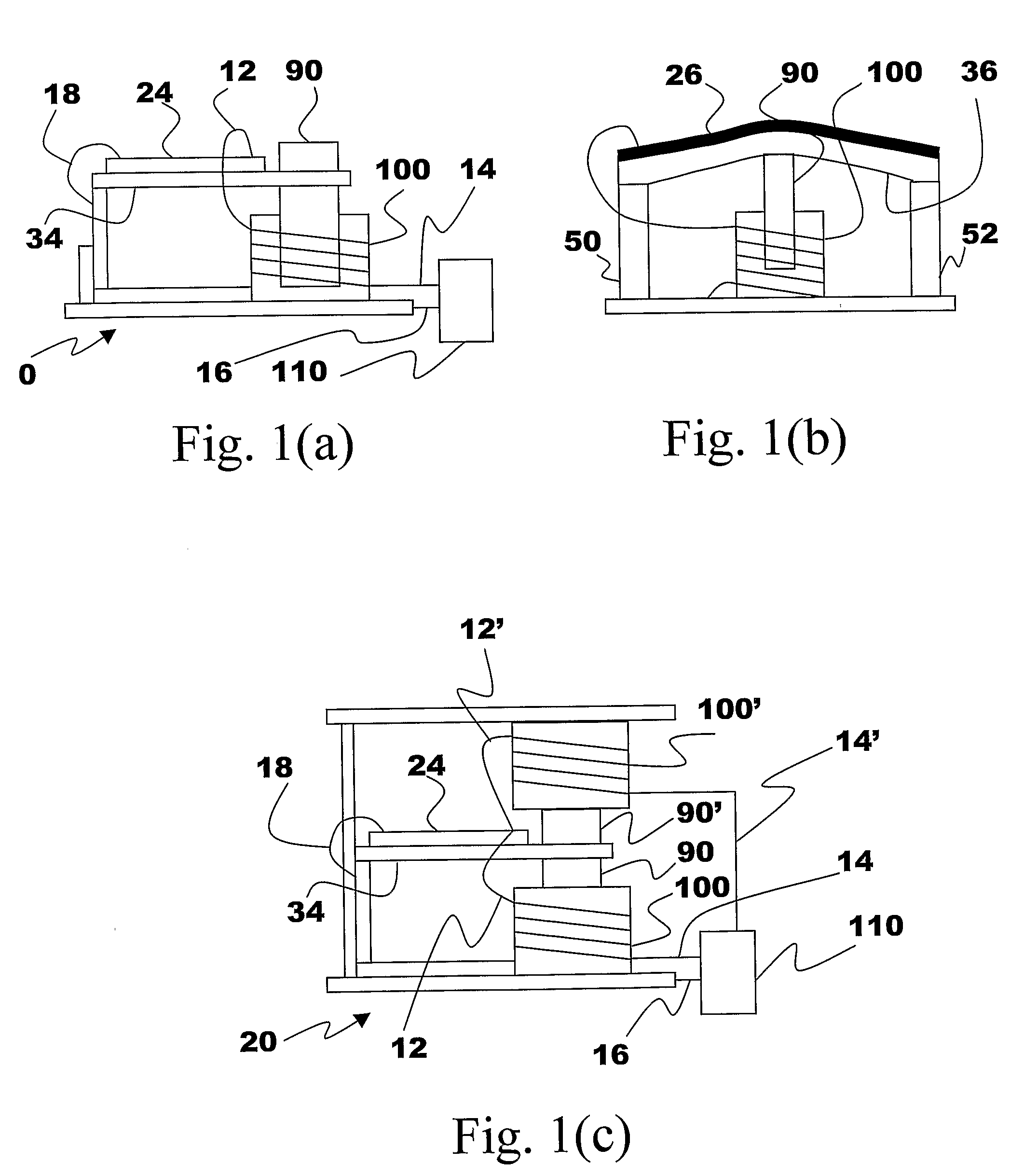
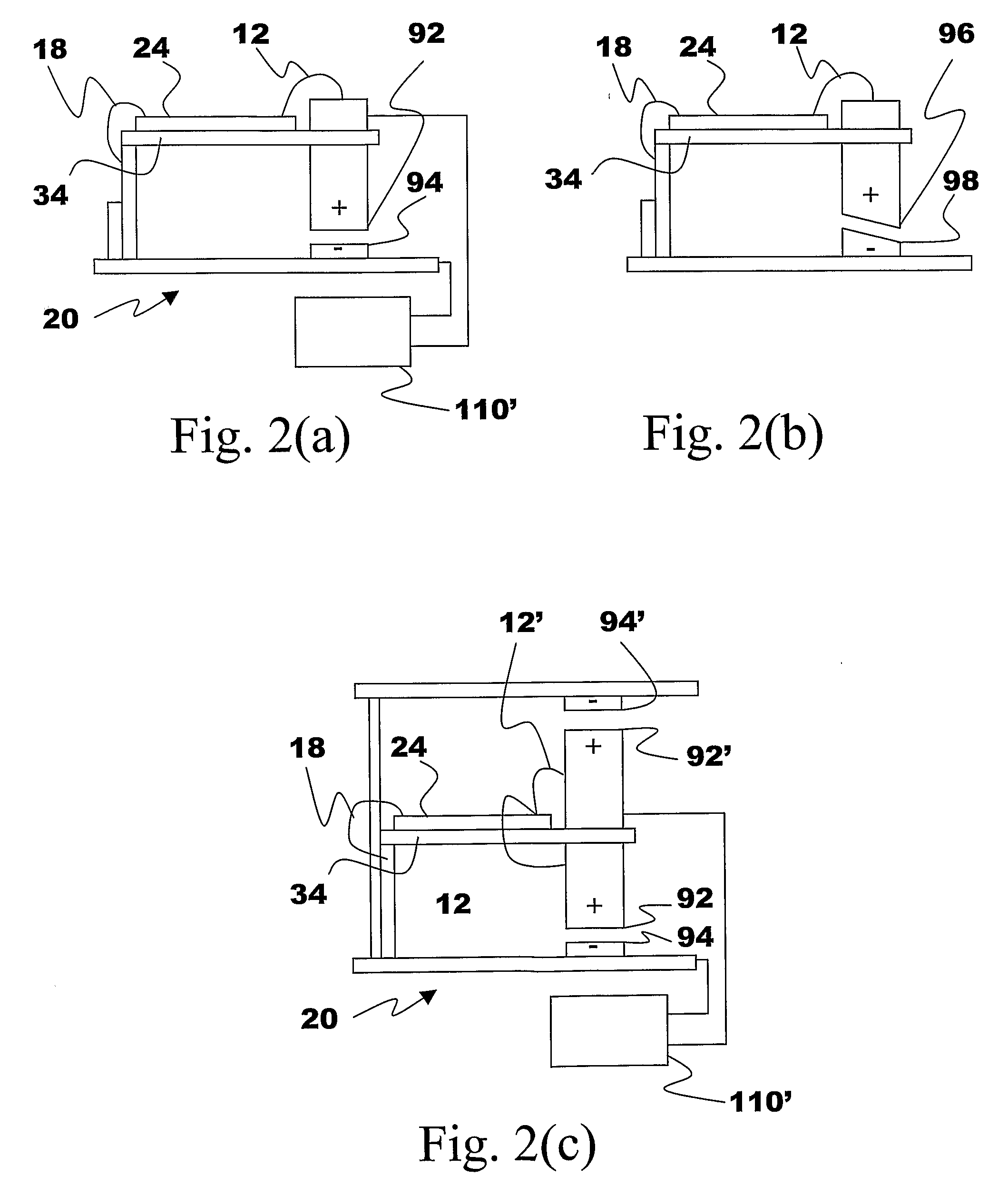
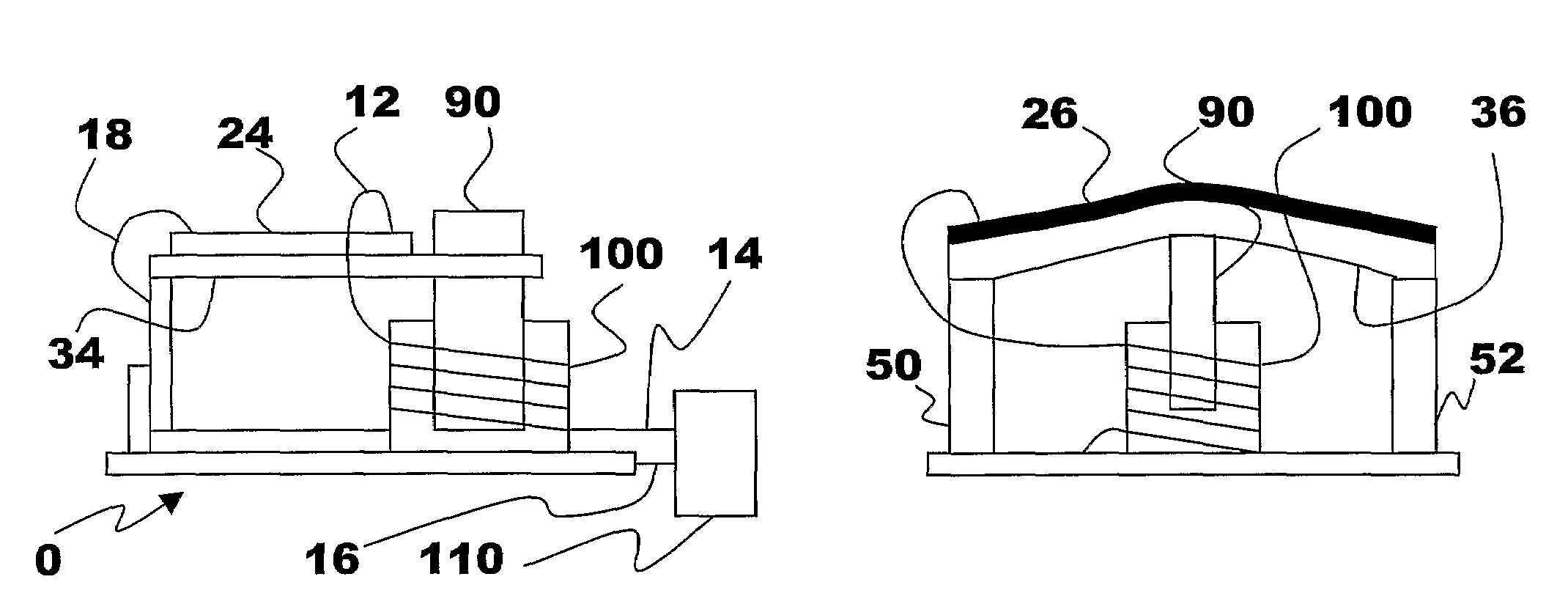
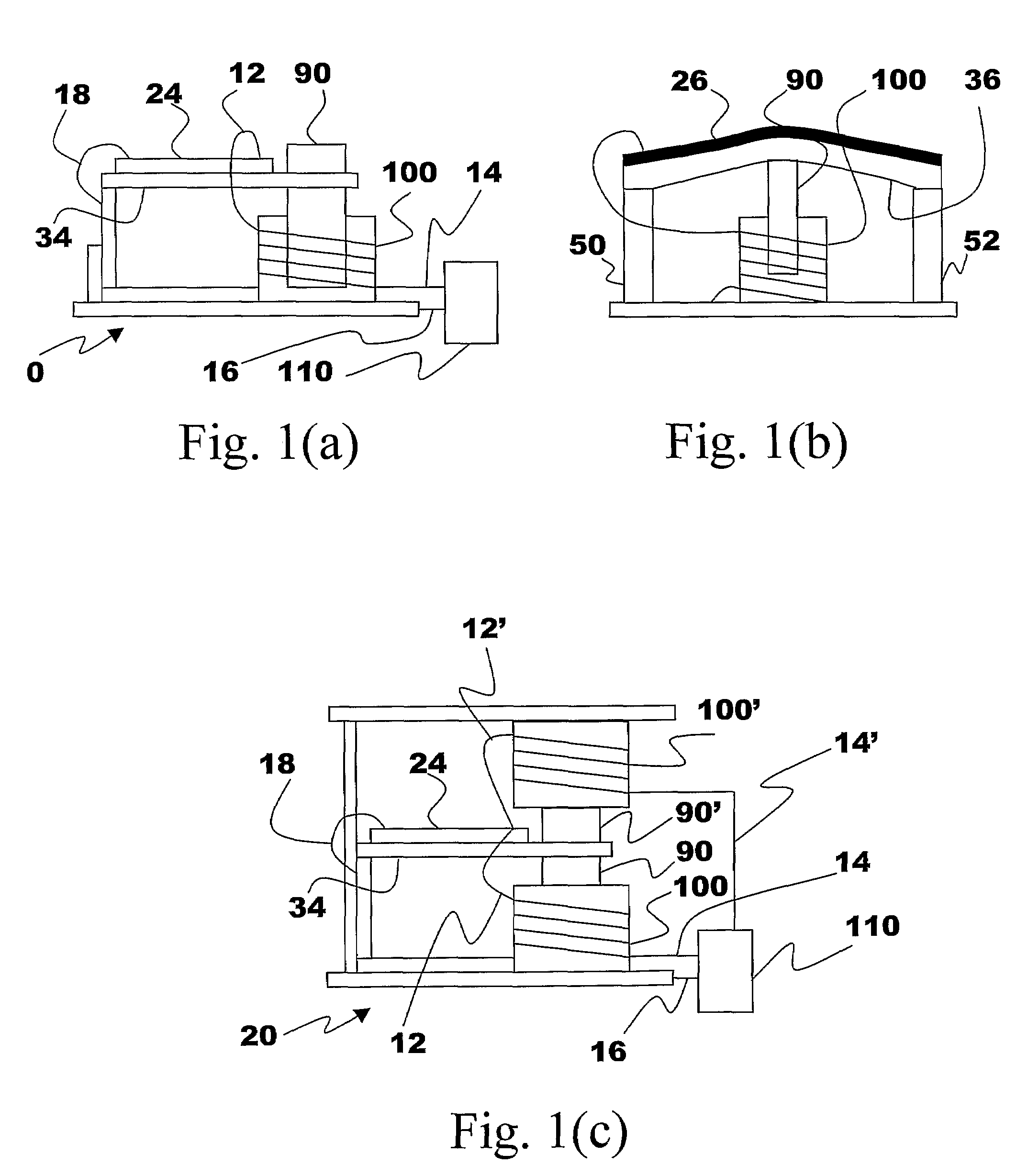
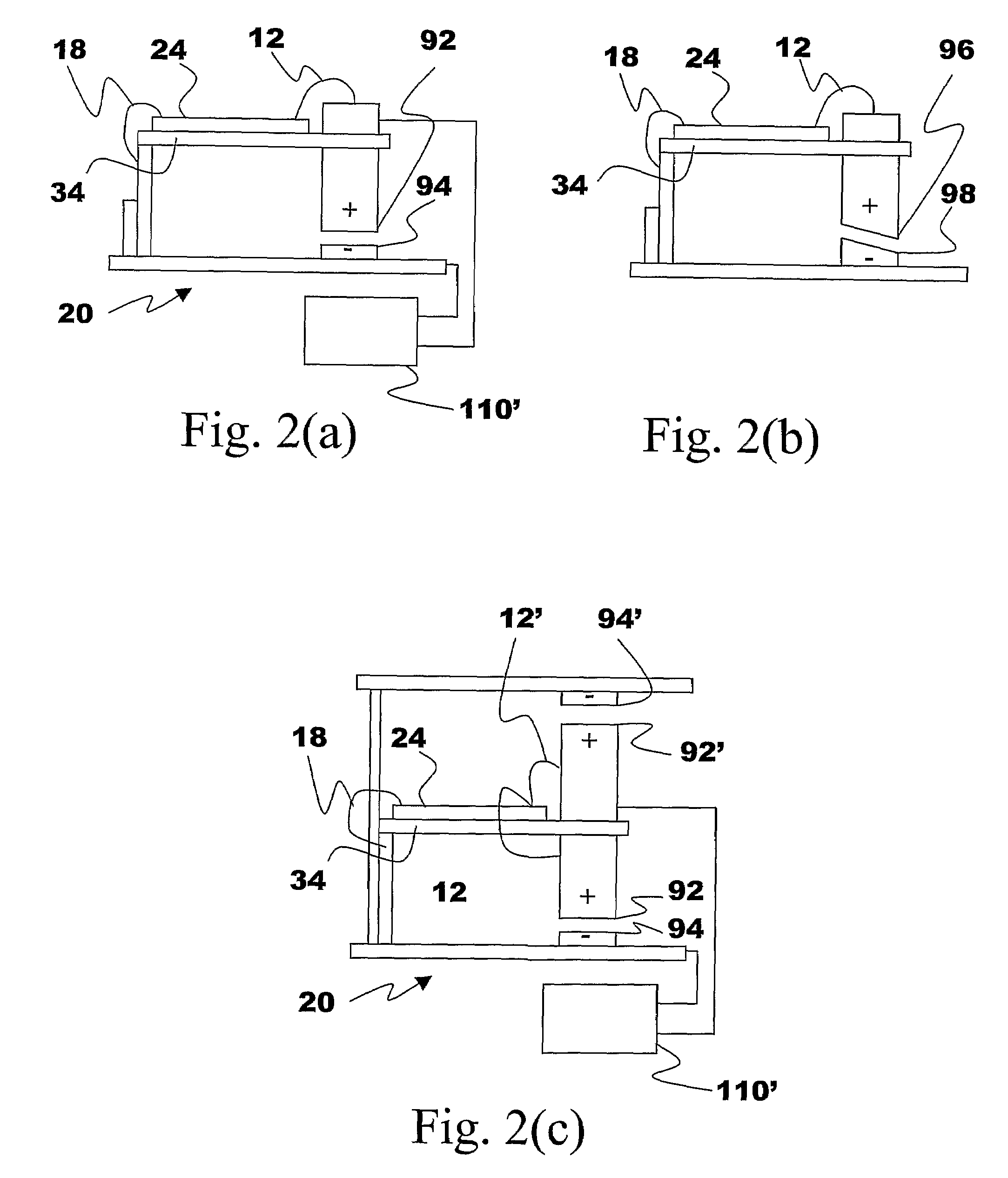
Energy Harvester with Adjustable Resonant Frequency
ActiveUS20080129147A1Facilitates great functionalityLess limitationPiezoelectric/electrostriction/magnetostriction machinesTyre measurementsCapacitanceElectricity
The present subject matter discloses devices, systems, and methodologies for harvesting power from environmentally induced vibrations. Piezoelectric devices (24) and structures are disclosed that may be employed in combination with electro-magnetic (100) or capacitive (92, 94) elements to enhance the power harvesting capabilities of the piezoelectric devices (24). The electromagnetic (100) and capacitive (92, 94) elements may be used to assist in maintaining system mechanical resonance in order to maximize energy harvesting capabilities. Power harvesting devices and systems in accordance with the subject technology may concurrently operate as sensors in motion sensitive applications thus providing self-powered monitoring capabilities.
Owner:MICHELIN RECH & TECH SA
Image processing apparatus and method
ActiveUS20110026600A1Appropriate performanceReduce filter effectColor television with pulse code modulationColor television with bandwidth reductionImaging processingControl unit
Provided is an image processing apparatus which includes a setting unit assigning a control block, which is a control unit of a filter process that is locally performed with respect to an image, to an initial position of the image determined based on a predetermined reference point; a movement unit moving the control block, which has been assigned to the initial position of the image by the setting unit, a to a position in which the result of the filter process is improved; and a filter processing unit performing the filter process for the respective control blocks which has been moved by the movement unit.
Owner:SONY CORP
Terminal and communication system
InactiveUS20070081512A1More complicated processComplicated processTime-division multiplexMultiprogramming arrangementsCommunications systemMethod selection
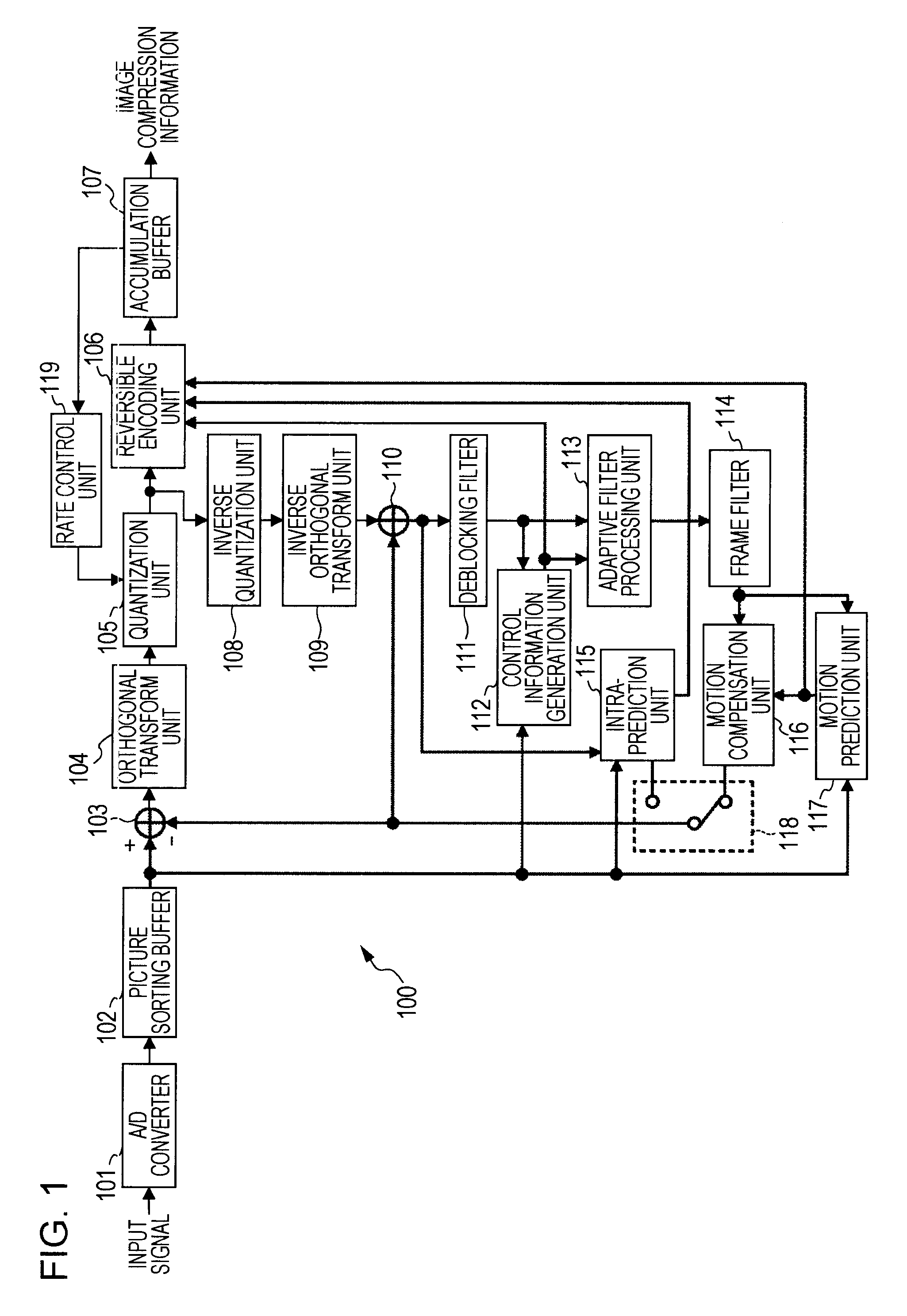
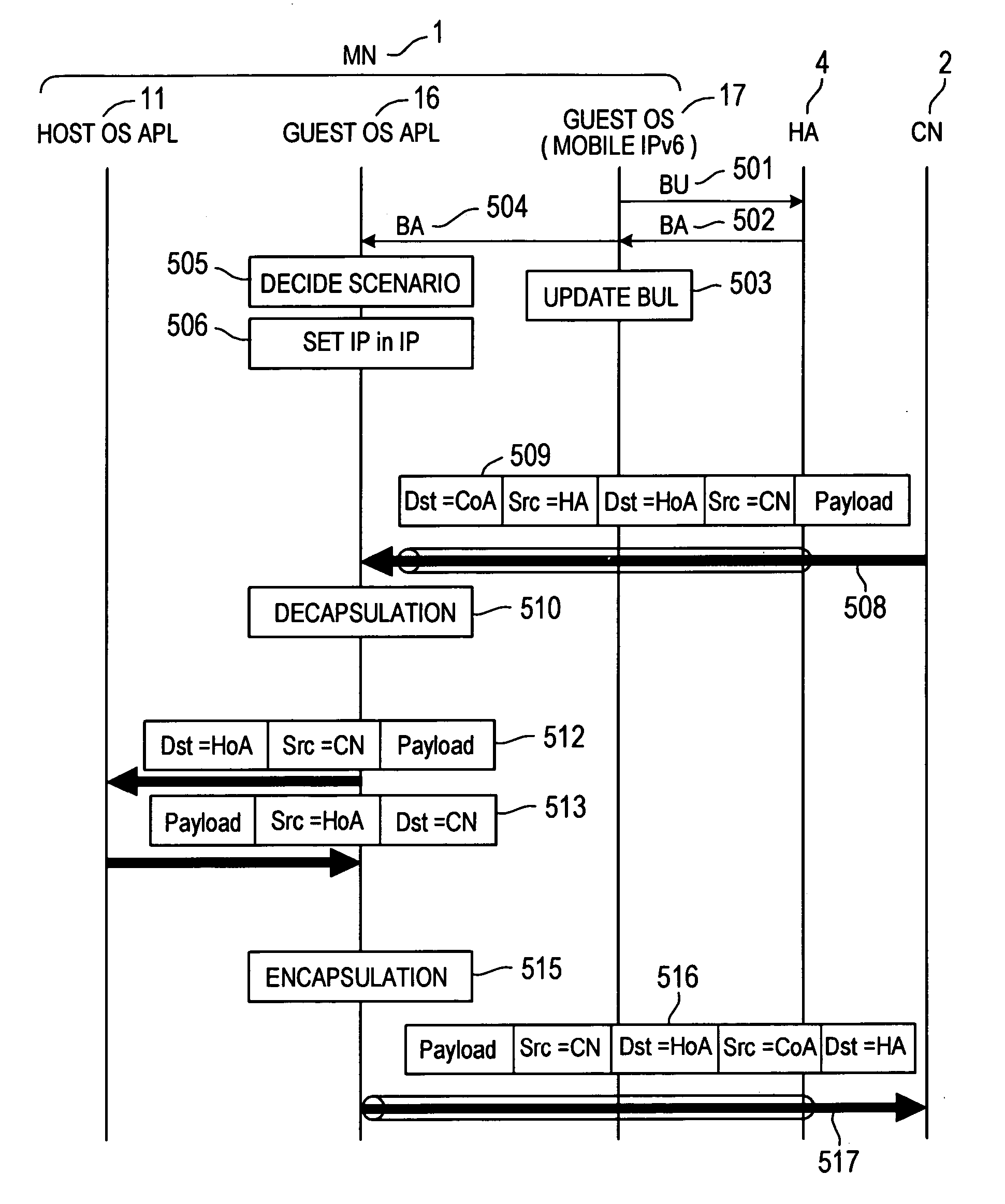
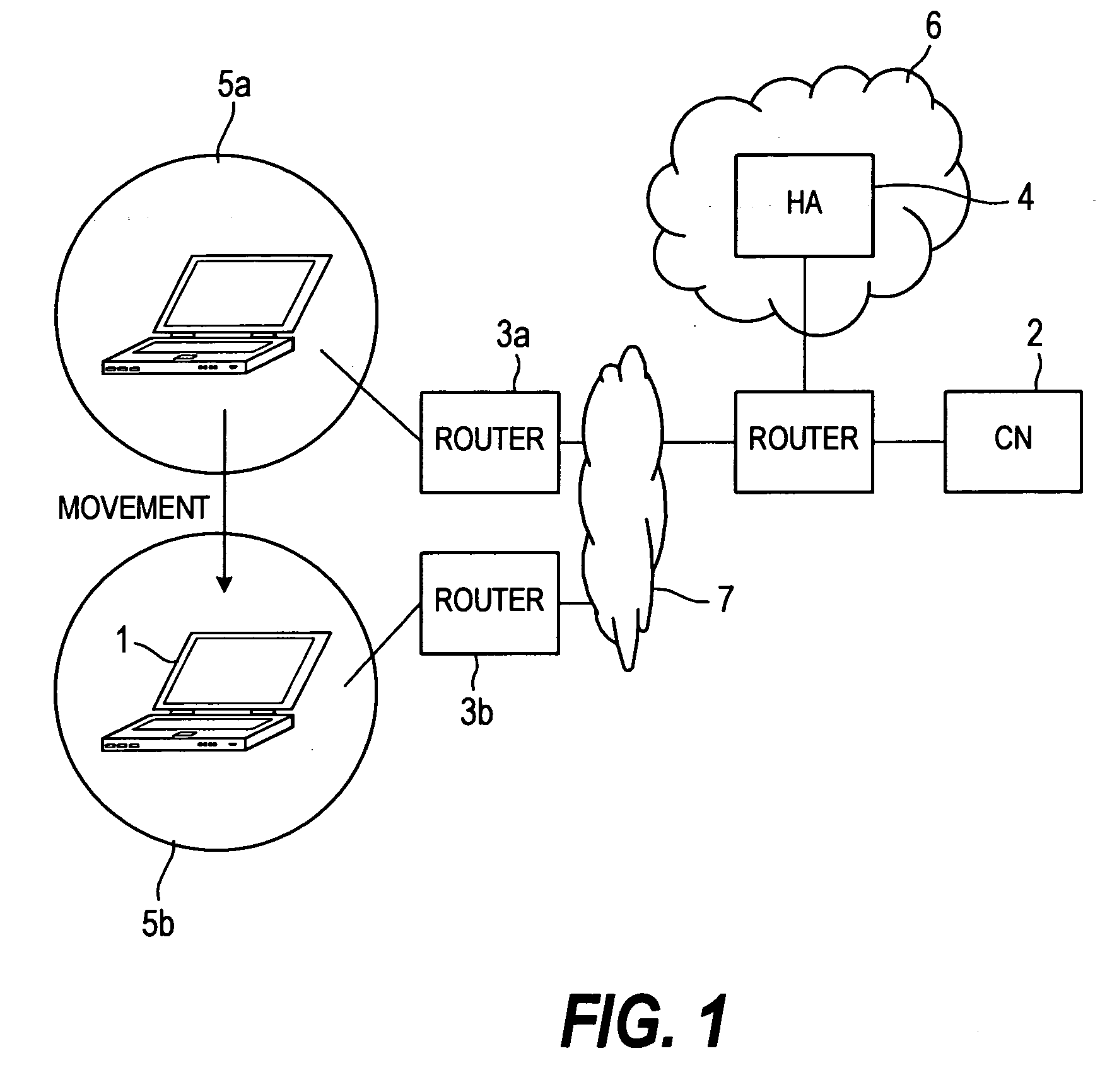
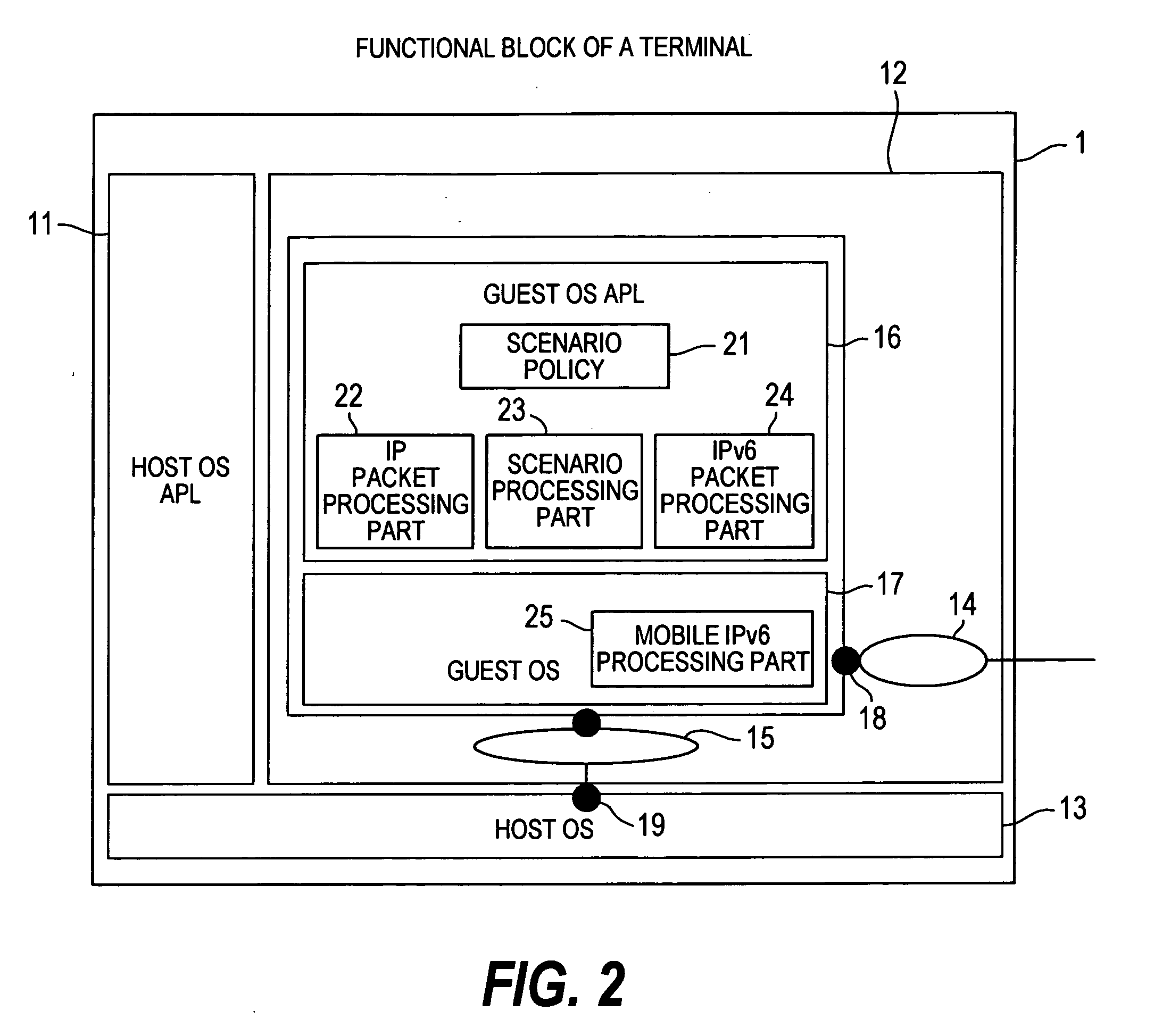
By assigning a fixed home address to an MN, Mobile IPv6 assures the arrival of each content at the MN 1. In order to allow the user to receive a Mobile IPv6 service, it is necessary to provide the MN 1 with a Mobile IPv6 function for making applications conform to IPv6. However, there are only few such MNs 1. In addition, the MN 1 does not have a function for implementing an IPsec process repeatedly on a packet transmitted to and received from another apparatus. A scenario processing port 23 employed in the MN 1 includes a means, which is used for selecting a process according to a communication method and carrying out the selected process when a response to a Mobile IPv6 location registration message is received. By providing the scenario processing port 23 with a means for selecting a communication method, a function can be added to the MN 1 with ease. In addition, by providing the MN 1 with a means for implementing an IPsec process a plurality of times, it is possible to provide a communication apparatus according to a security management configuration.
Owner:HITACHI LTD
Generating sets of tailored laser pulses
ActiveUS20050041976A1Improve removal qualityComplicated processLaser detailsSemiconductor/solid-state device detailsAudio power amplifierMaster oscillator
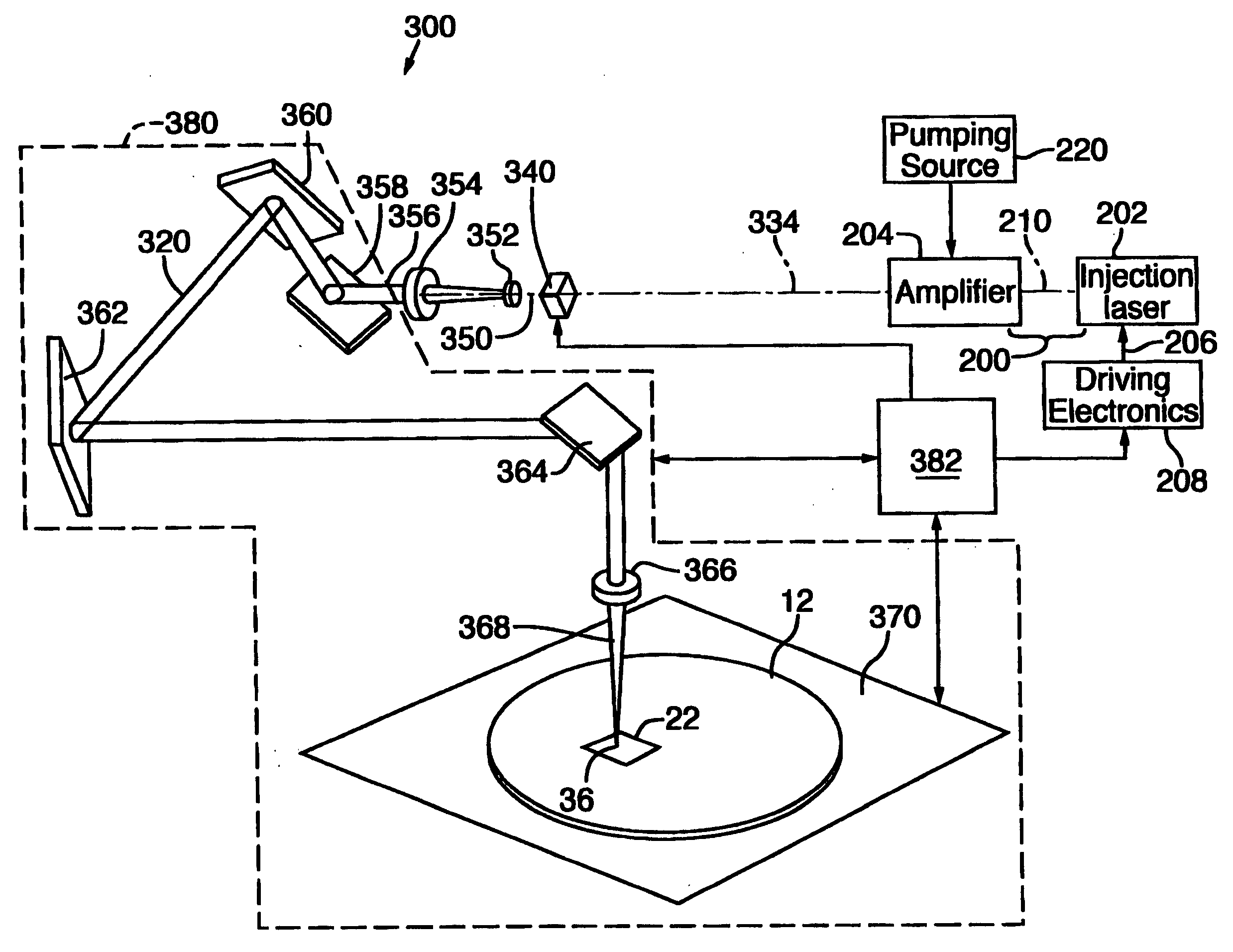
In a master oscillator power amplifier, a driver (208) of a diode laser (202) is specially controlled to generate a set of two or more injection laser pulses that are injected into a power amplifier (204) operated in an unsaturated state to generate a set (50) of laser pulses (52) that replicate the temporal power profile of the injection laser pulses to remove a conductive link (22) and / or its overlying passivation layer (44) in a memory or other IC chip. Each set (50) includes at least one specially tailored pulse (52) and / or two or more pulses (50) having different temporal power profiles. The duration of the set (50) is short enough to be treated as a single “pulse” by conventional positioning systems (380) to perform on-the-fly link removal without stopping.
Owner:ELECTRO SCI IND INC
Imaging-guided anesthesia injection systems and methods
InactiveUS20130041258A1Precise deliveryPatient benefitUltrasonic/sonic/infrasonic diagnosticsAutomatic syringesAnatomical structuresDisplay device
Devices and systems for injecting fluids, such as anesthetics, to or near nerve tissue or other targeted anatomical location are disclosed herein. A conduit is generally configured to place the fluid delivery module in fluid communication with a needle that is configured to be inserted into the patient's anatomy. One or more medicaments (e.g., anesthetics) and / or other materials contained within containers (e.g., vials) that are secured to the injection system can be selectively delivered into an anatomy through the needle. Nerve stimulation and / or imaging technologies (e.g., ultrasound) can be used to locate a targeted anatomical location. Aspiration can be used to confirm needle location. An overlay on the imaging display can include, in addition to real-time imaging data, data and other information relating to back pressure at or near the needle tip, volumes or other amounts of fluids delivered by and remaining within the system, stimulation level and / or the like.
Owner:CARTICEPT MEDICAL
Speech recognition apparatus and speech recognition method
ActiveUS7310601B2Appropriate performanceComplicated processSpeech recognitionSpeech identificationSpeech sound
Owner:PANASONIC INTELLECTUAL PROPERTY CORP OF AMERICA
Conductive sheet, method for using conductive sheet, and capacitive touch panel
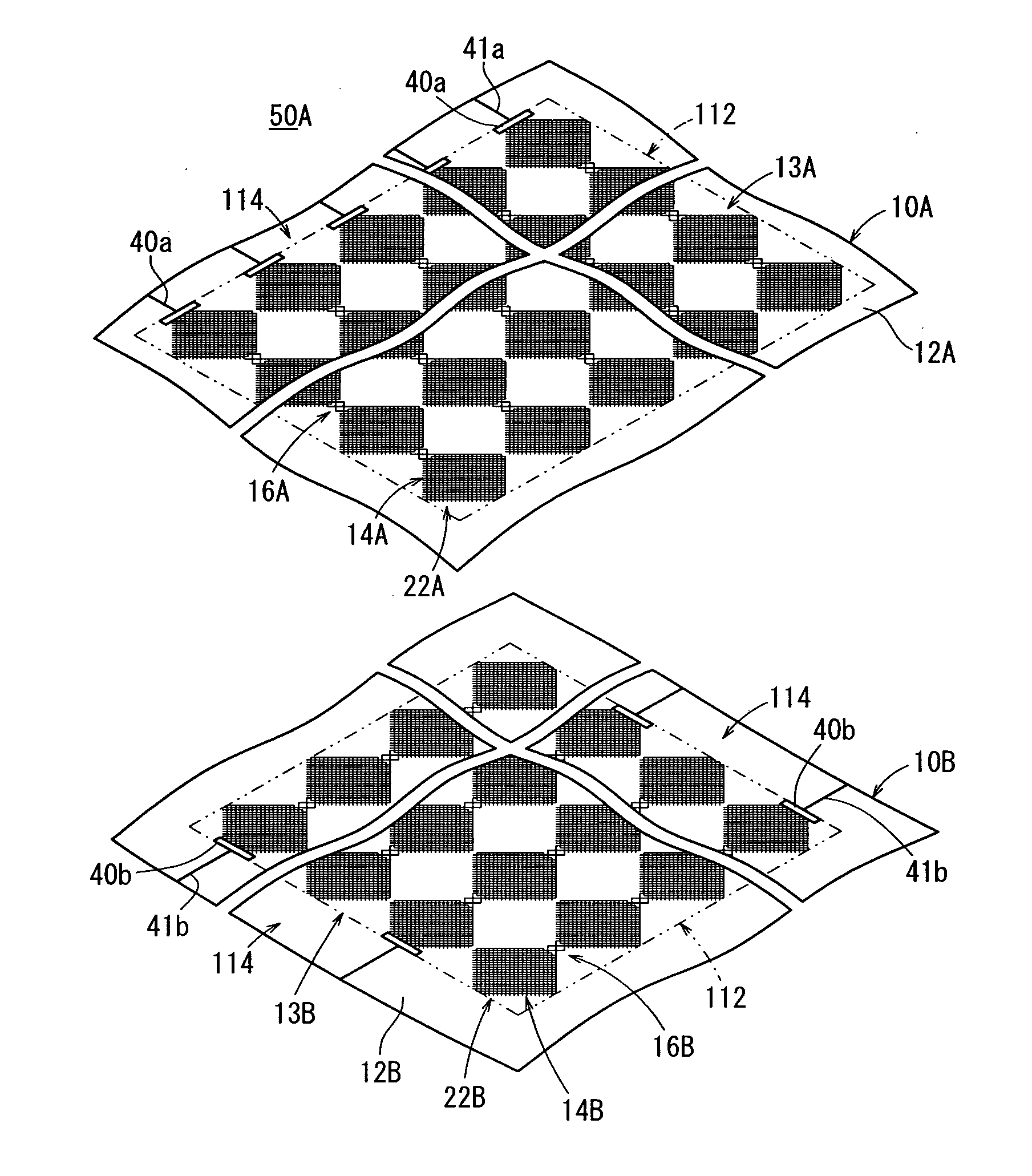
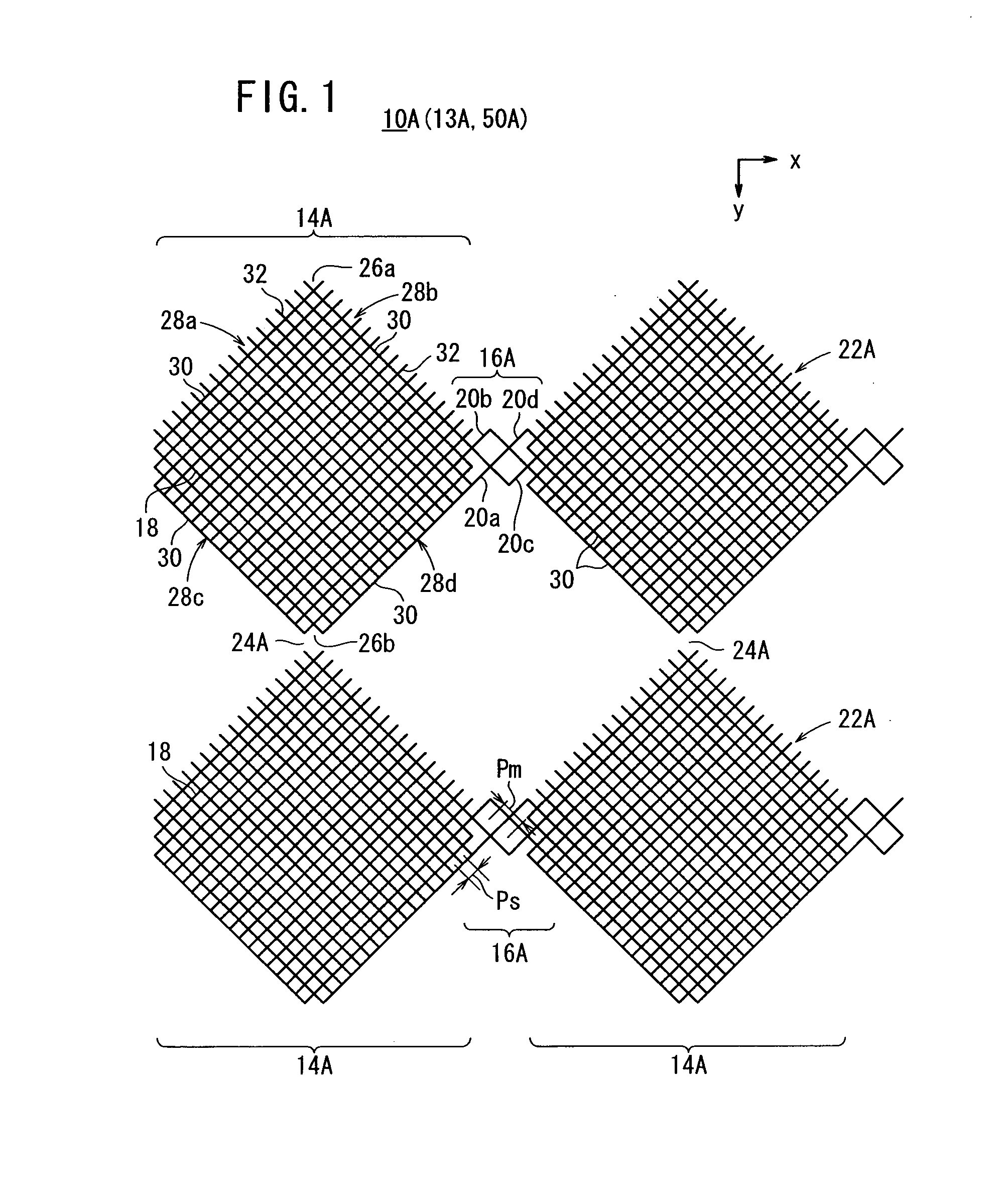
ActiveUS20120118614A1Serious disadvantageImprove visibilityConductive layers on insulating-supportsElectrical connection printed elementsEngineeringTouch panel
A conductive Sheet, a method for using conductive sheet, and a capacitive touch panel are provided. A first conductive sheet contains two or more conductive first large lattices and a first connection for electrically connecting the adjacent first large lattices on a first transparent substrate. The first large lattices each contain a combination of two or more small lattices, the first connection contains one or more medium lattices (a first medium lattice to a fourth medium lattice), and the pitch of the medium lattices is n times larger than that of the small lattices (in which n is a real number larger than 1).
Owner:FUJIFILM CORP
Authenticating banknotes or other physical objects
InactiveUS20090008924A1Improve securityEasy to measureRadiation pyrometryOther printing matterComputer sciencePhysical property
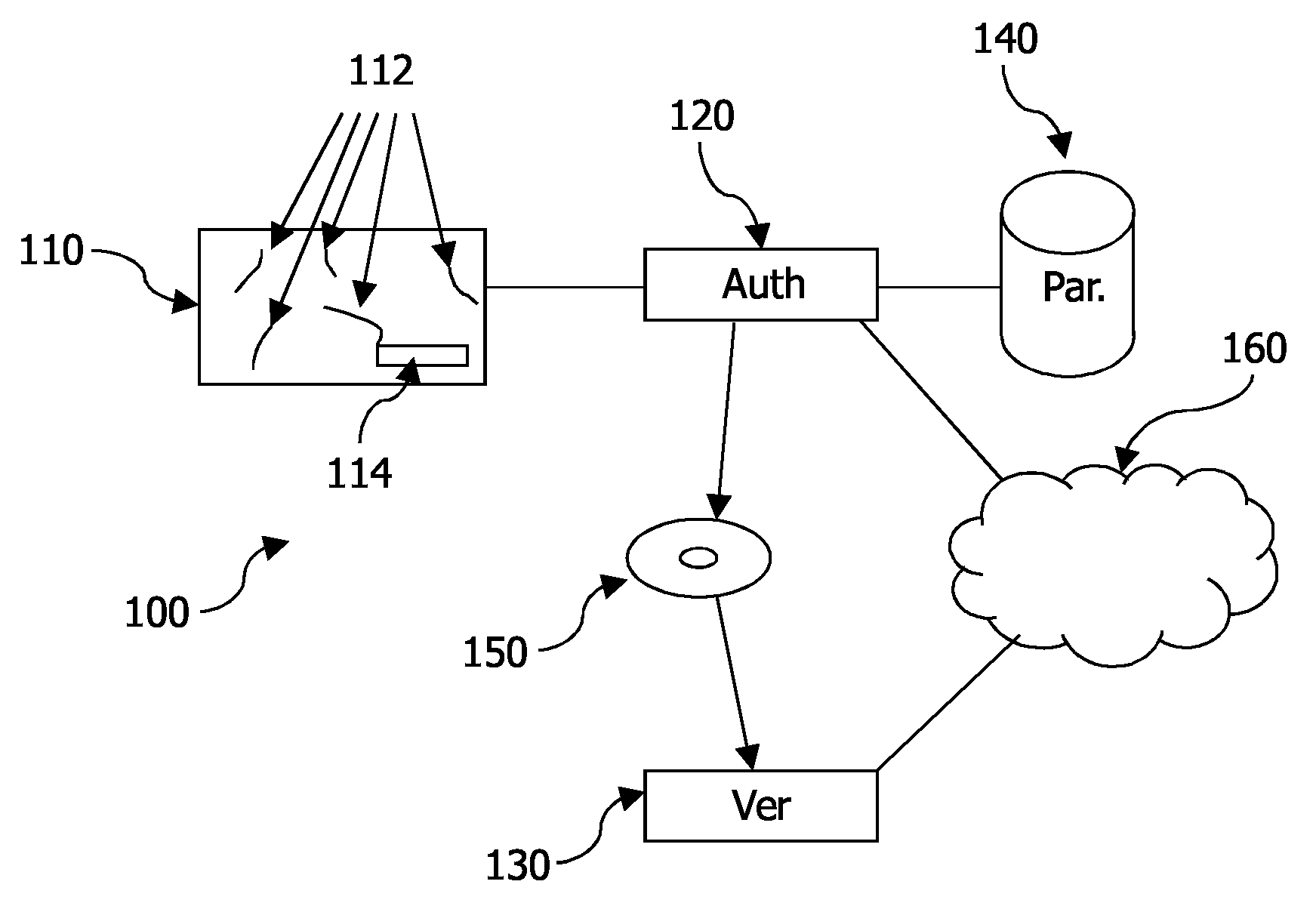
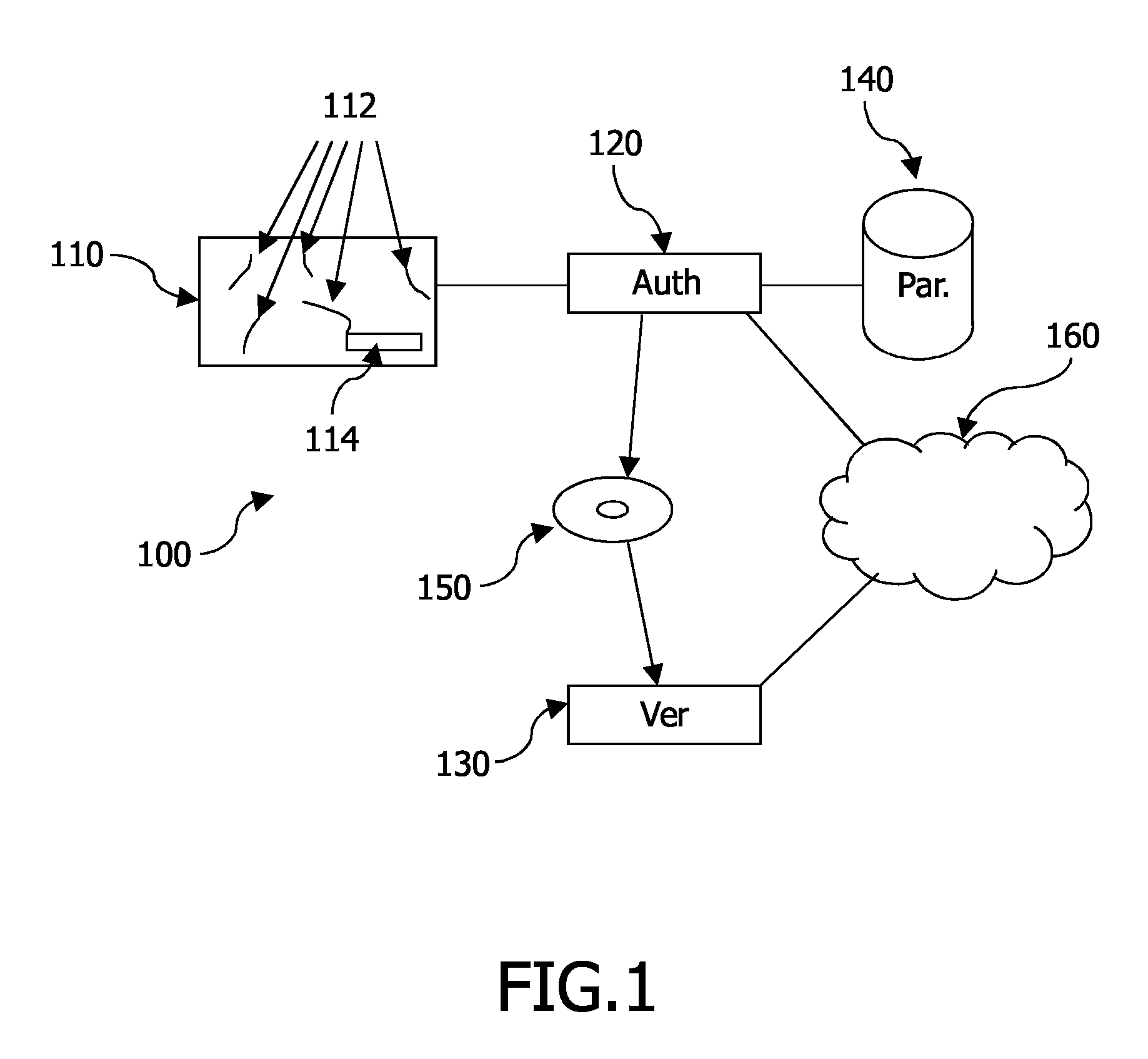
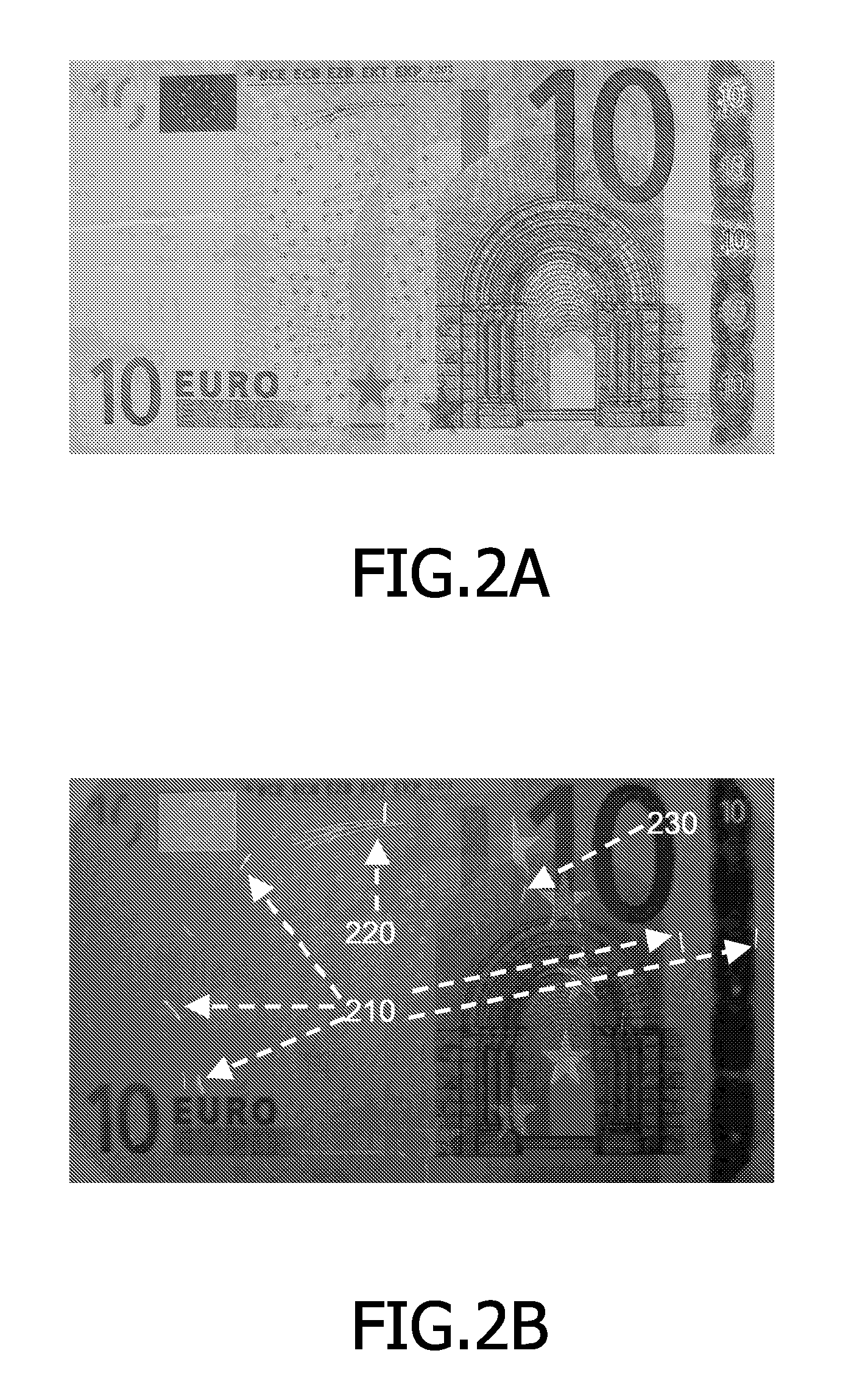
A system 100 for authenticating a physical product 110, such as a banknote, including at least one physical product and a verification device 130. The physical product including a random distribution of a plurality of physically detectable particles 112 in a substrate of the product. In association with the physical product, a digital representation (114) is stored (‘stored representation’) of measured physical properties of the particles including an actual distribution of at least some of the particles, where the physical properties are measured through reflection and transmission. The verification device includes a measurement unit 450 for determining a digital representation (‘measured representation’) based on measurements of physical properties of the particles, including an actual distribution of at least some of the particles, through reflection and transmission; and a comparison unit 470 for comparing the measured representation with the stored representation.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Energy harvester with adjustable resonant frequency
ActiveUS7471033B2Compact manufacturingProcess compatiblePiezoelectric/electrostriction/magnetostriction machinesTyre measurementsCapacitanceElectricity
The present subject matter discloses devices, systems, and methodologies for harvesting power from environmentally induced vibrations. Piezoelectric devices (24) and structures are disclosed that may be employed in combination with electro-magnetic (100) or capacitive (92, 94) elements to enhance the power harvesting capabilities of the piezoelectric devices (24). The electromagnetic (100) and capacitive (92, 94) elements may be used to assist in maintaining system mechanical resonance in order to maximize energy harvesting capabilities. Power harvesting devices and systems in accordance with the subject technology may concurrently operate as sensors in motion sensitive applications thus providing self-powered monitoring capabilities.
Owner:MICHELIN & CO CIE GEN DES ESTAB MICHELIN
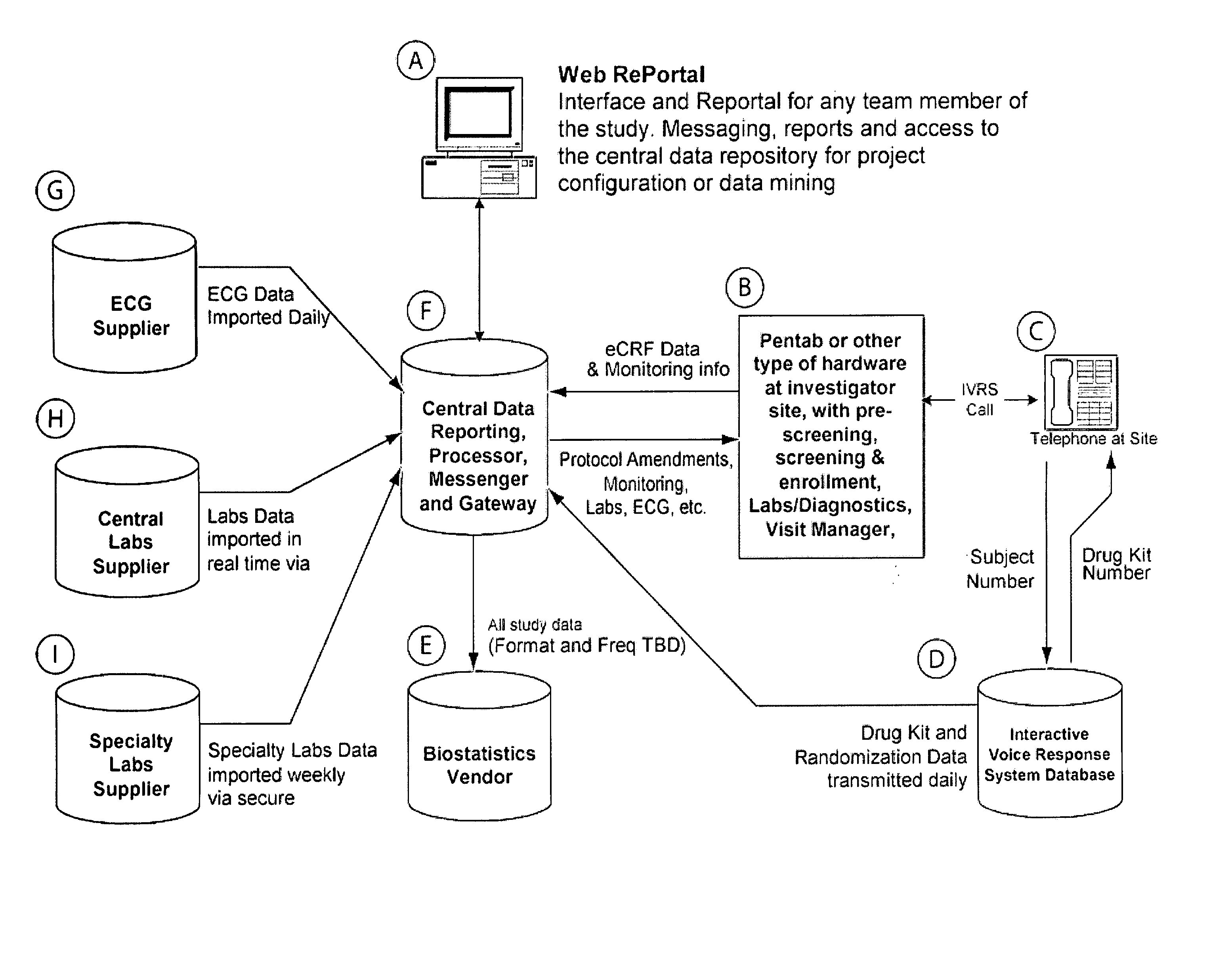
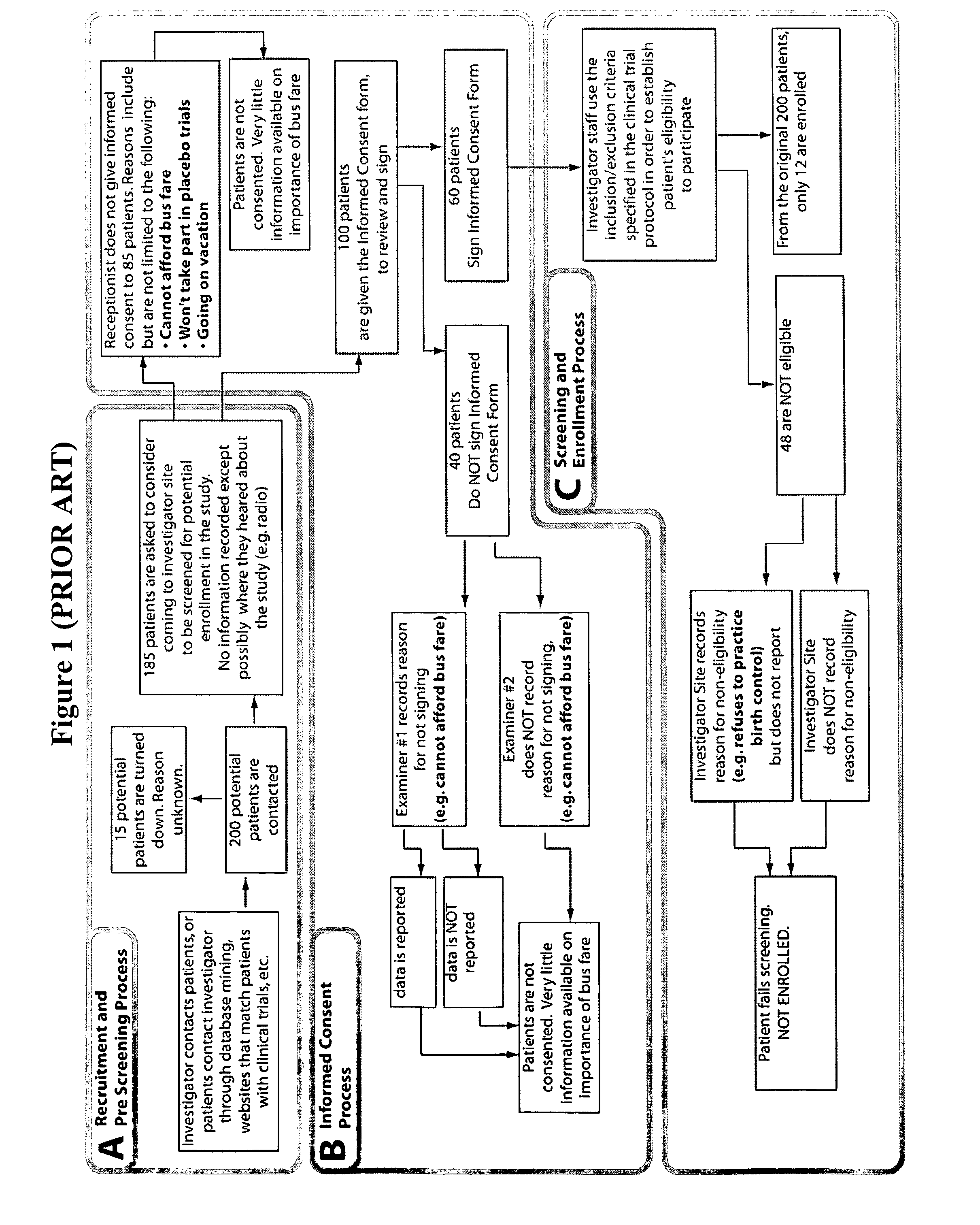
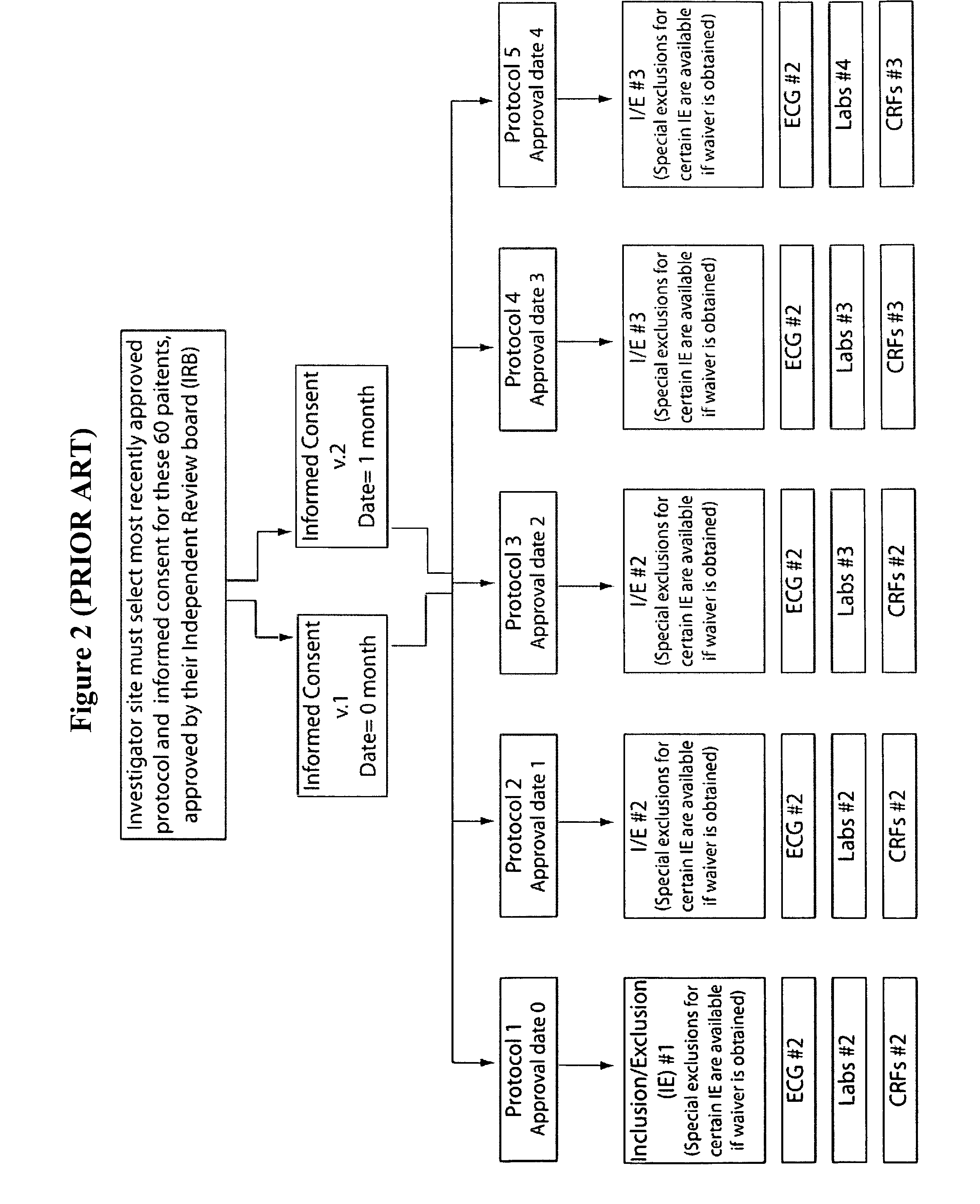
Method and apparatus for screening, enrollment and management of patients in clinical trials
InactiveUS20070067189A1Simple speedImproves of enrollment processData processing applicationsComputer-assisted medical data acquisitionDiagnostic testData center
A computer-implemented method of tracking patient data in a clinical trial is provided. The clinical trial has one or more investigative sites which perform patient screening and enrollment for the clinical trial, one or more diagnostic sites which perform analysis on one or more patient diagnostic tests ordered by an investigative site and generate analysis results, and a centralized data center in electronic communication with the one or more investigative sites and the one or more diagnostic sites. Each investigative site is provided with a user interface display screen for allowing a user at the investigative site to enter data regarding patients who have been screened for the clinical trial and patients who have been enrolled in the clinical trial. The data from each of the investigative sites is electronically communicated to the centralized data center. Also, the analysis results from each of the diagnostic sites are electronically communicated to the centralized data center. The centralized data center consolidates the data and analysis results from each of the sites and provides one or more status reports regarding the patients for whom data and analysis results were received from the one or more investigative sites and the one or more diagnostic sites.
Owner:NUMODA TECH
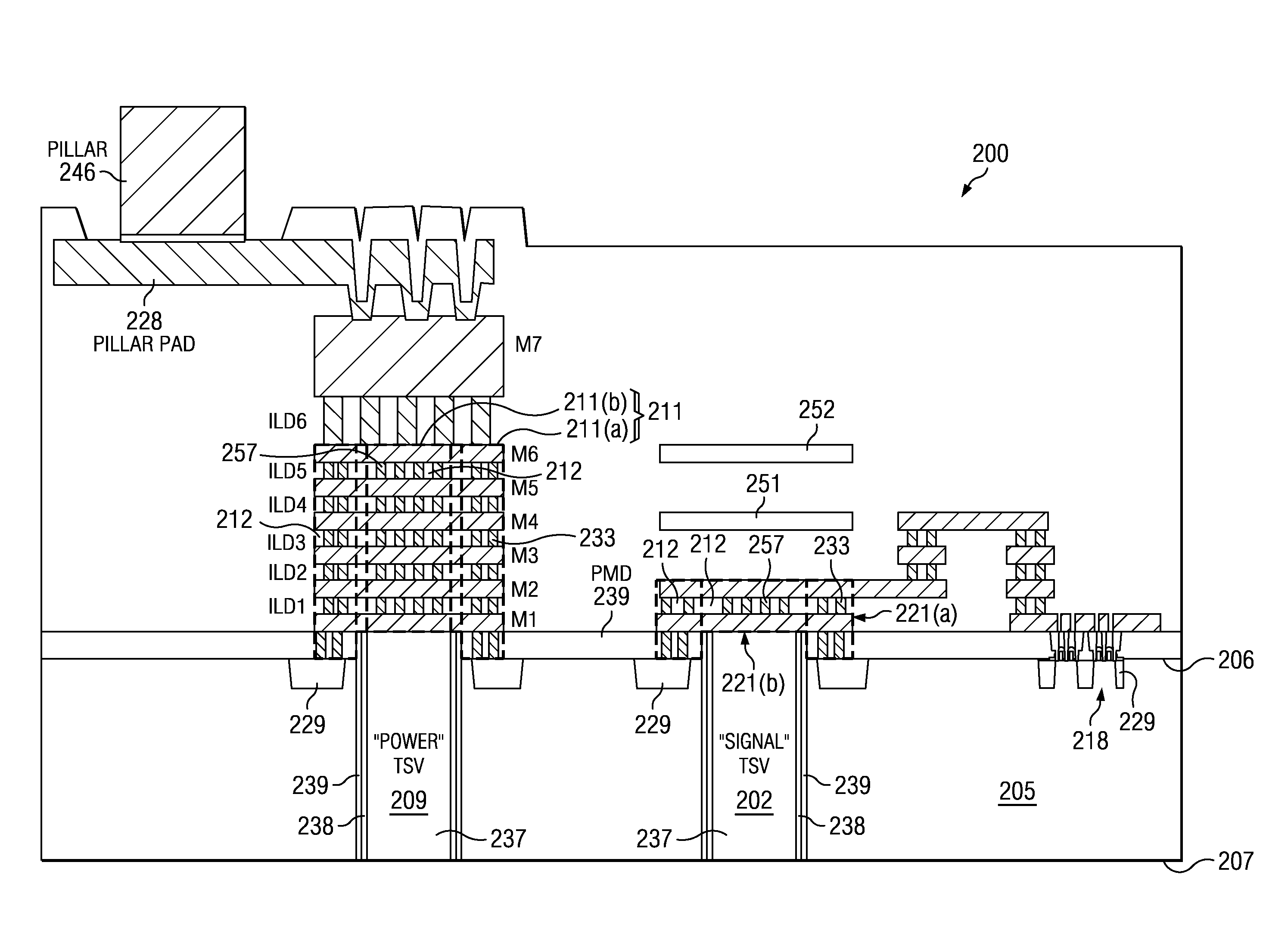
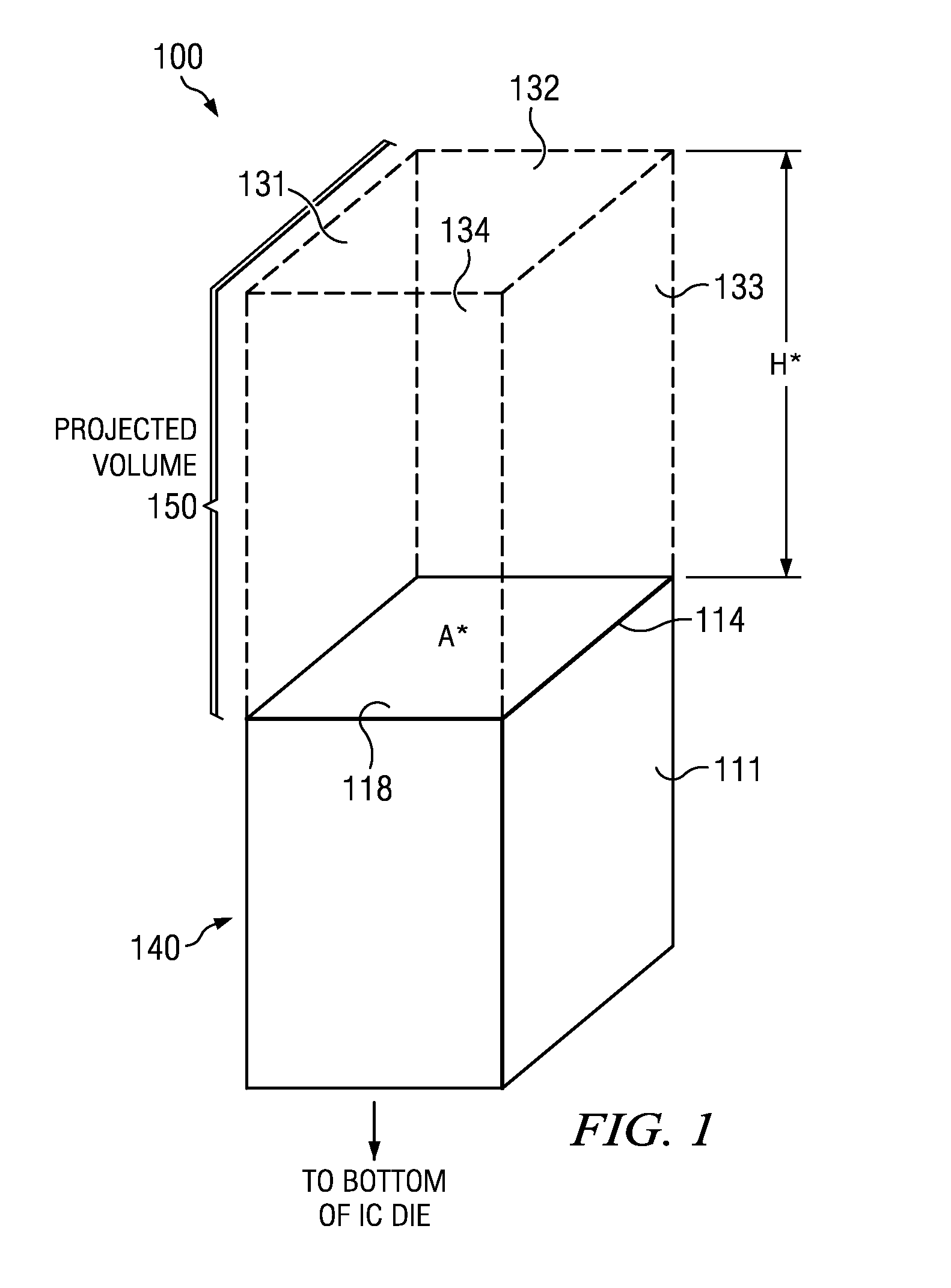
Integrated circuit (IC) having tsvs with dielectric crack suppression structures
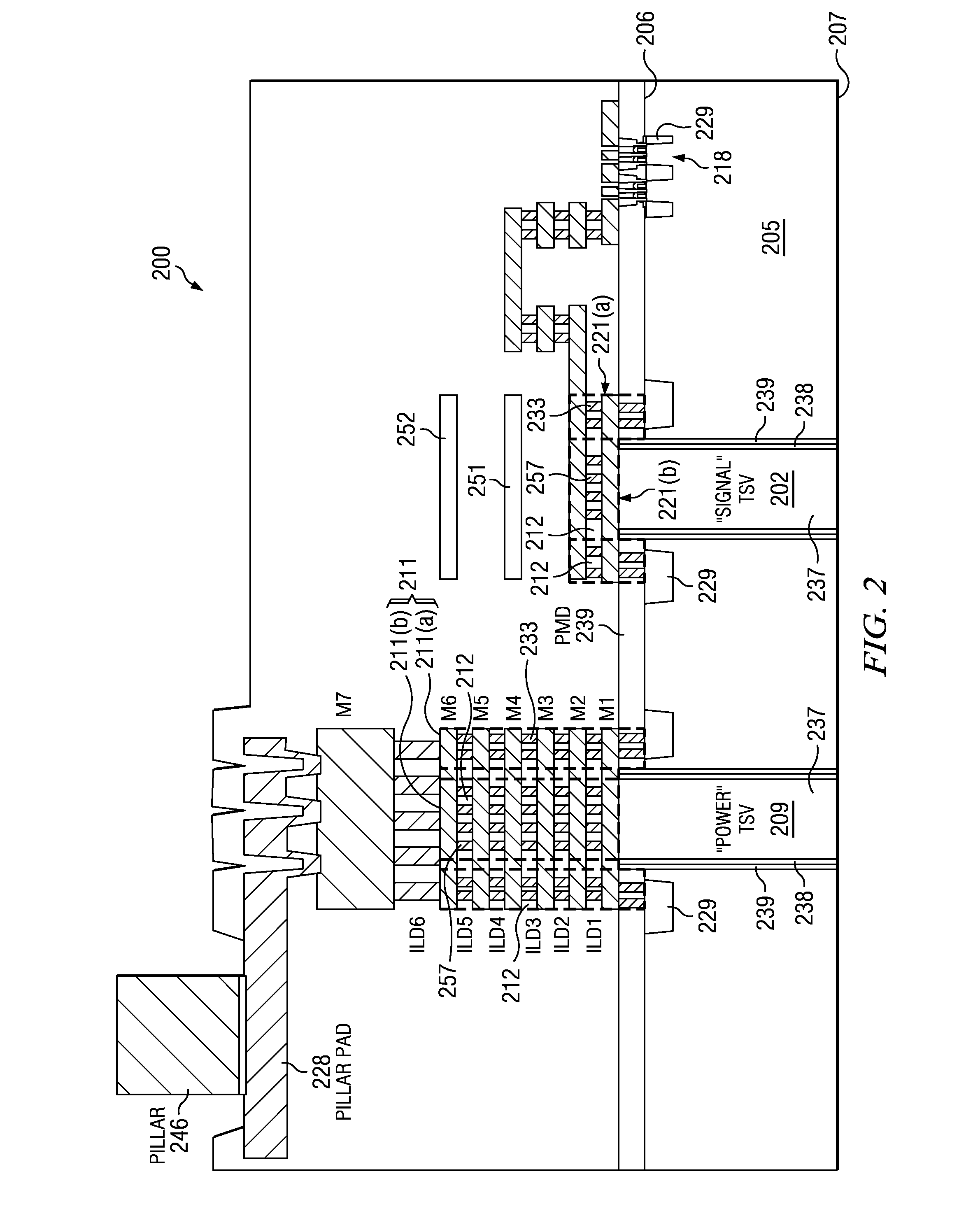
ActiveUS20110031581A1Reduce the amount requiredTSV resistanceSemiconductor/solid-state device detailsSolid-state devicesCrazingMetal interconnect
An IC includes a substrate having a semiconductor top surface, a plurality of metal interconnect levels having inter-level dielectric (ILD) layers therebetween on the top surface, and a bottom surface. A plurality of through substrate vias (TSVs) extend from a TSV terminating metal interconnect level downward to the bottom surface. The plurality of TSVs include an electrically conductive filler material surrounded by a dielectric liner that define a projected volume. The projected volume includes a projected area over the electrically conductive filler material and a projected height extending upwards from the TSV terminating metal interconnect level to a metal interconnect level above, and a projected sidewall surface along sidewalls of the projected volume. A crack suppression structure (CSS) protects TSVs and includes a lateral CSS portion that is positioned lateral to the projected volume and encloses at least 80% of the projected sidewall surface.
Owner:TEXAS INSTR INC
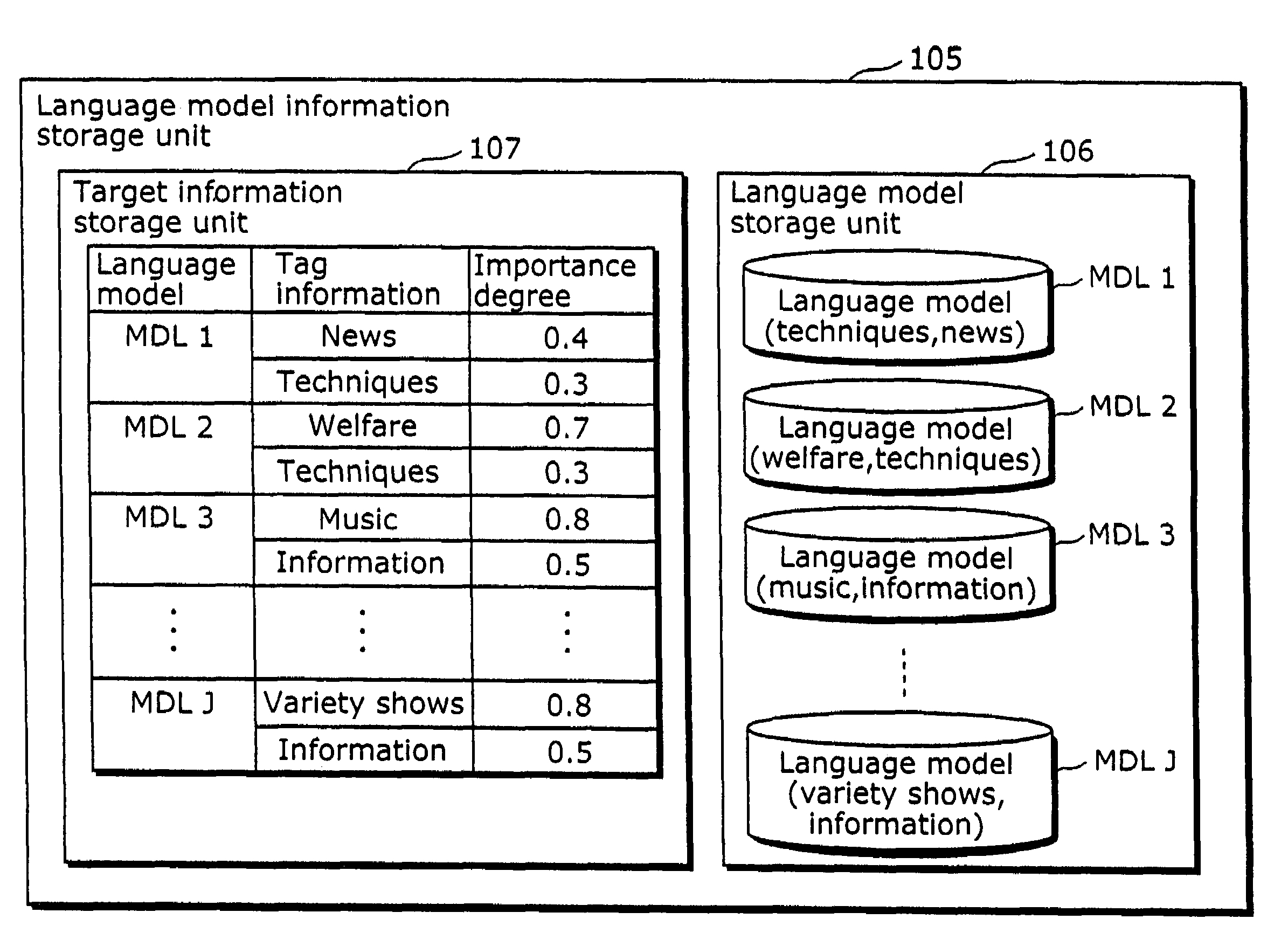
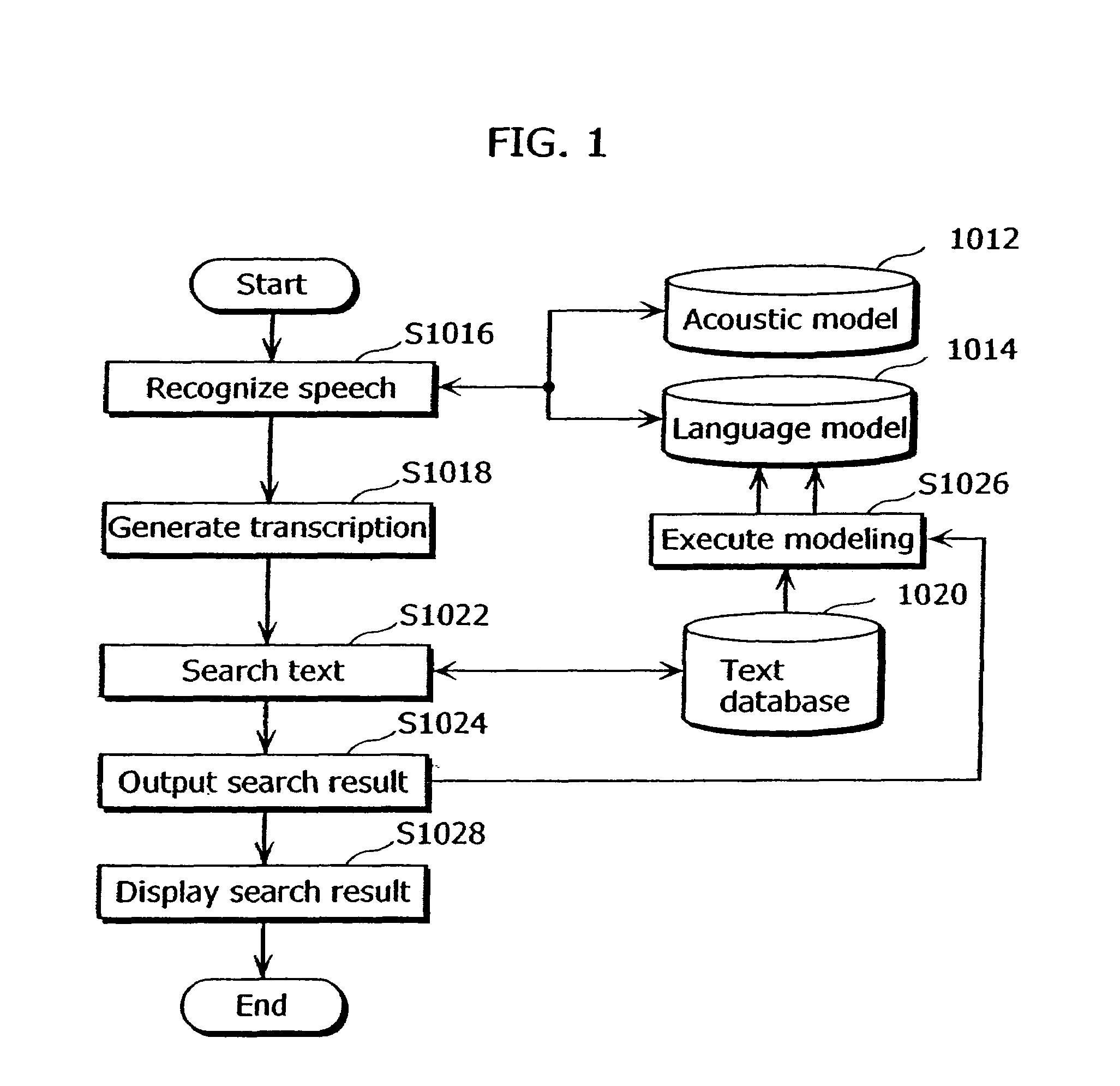
Speech recognition apparatus and speech recognition method
ActiveUS20060100876A1Appropriate performanceComplicated processSpeech recognitionSpeech identificationSpeech sound
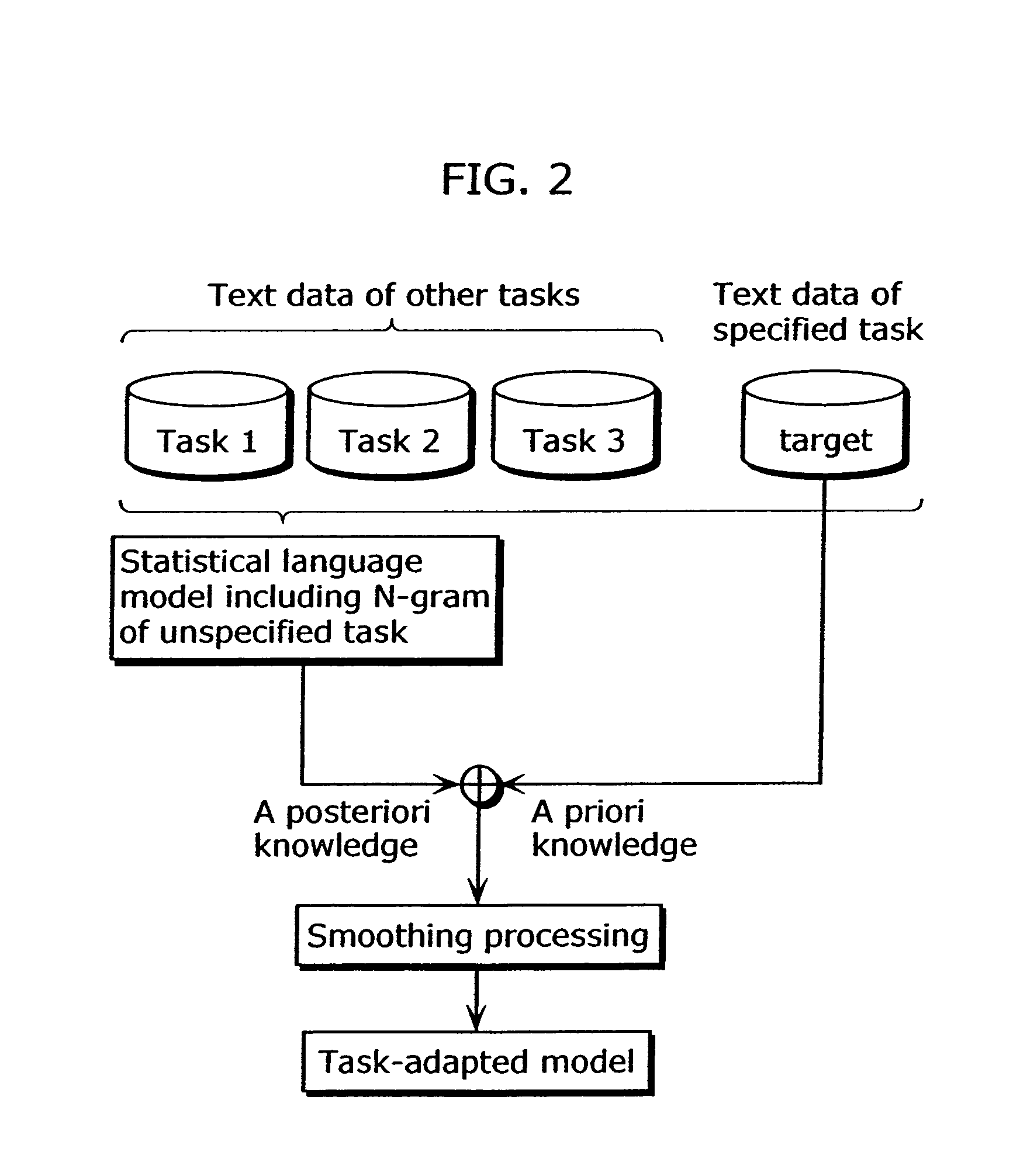
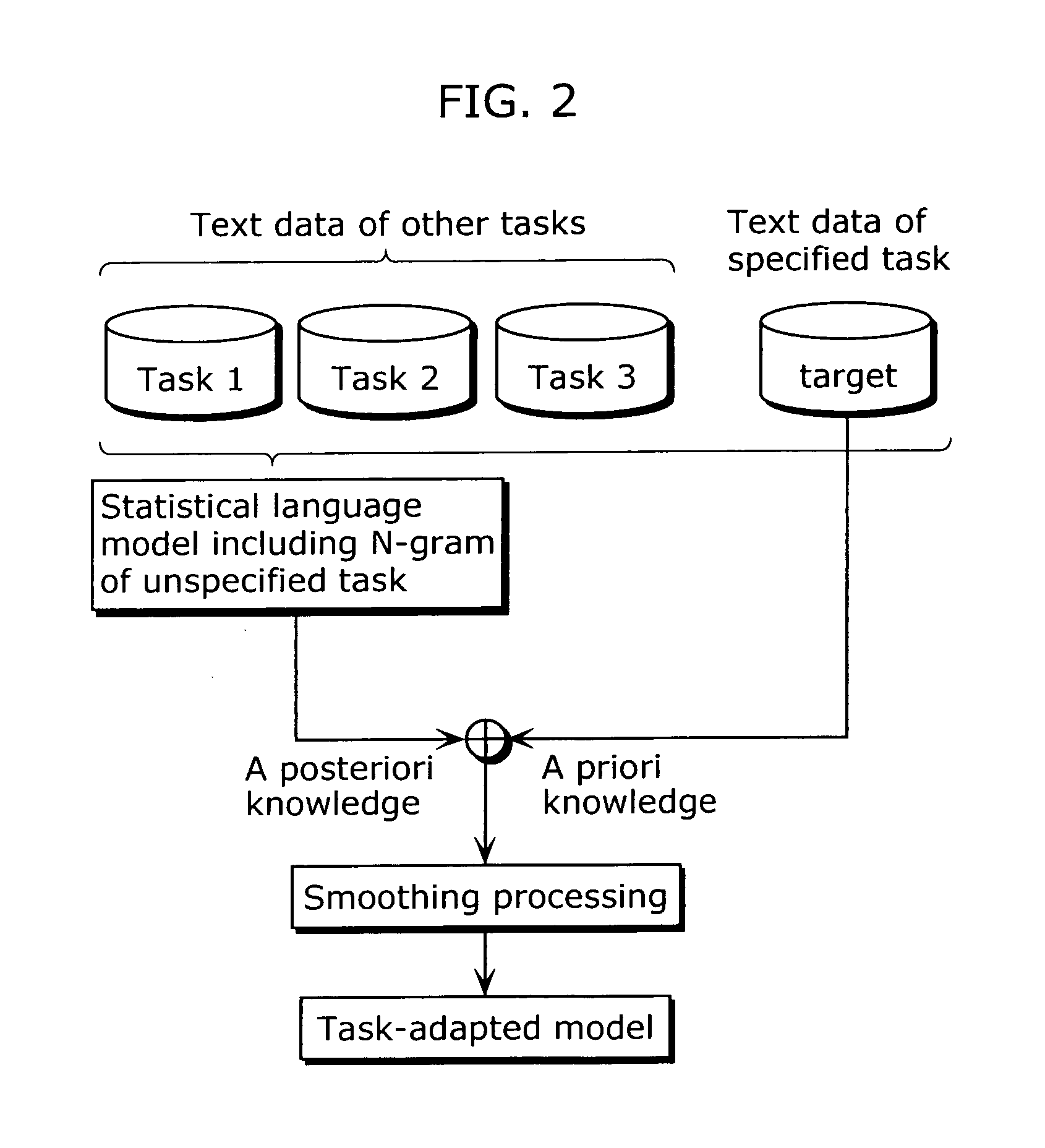
To provide a speech recognition apparatus which appropriately performs speech recognition by generating, in real time, language models adapted to a new topic even in the case where topics are changed. The speech recognition apparatus includes: a word specification unit for obtaining and specifying a word; a language model information storage unit for storing language models for recognizing speech and the respectively corresponding pieces of tag information; a combination coefficient calculation unit for calculating the weights of the respective language models, as combination coefficients, according to the word obtained by the word specification unit, based on the relevance degree between the word obtained by the word specification unit and the tag information of each language model; a language probability calculation unit for calculating the probabilities of word appearance by combining the respective language models according to the calculated combination coefficients; and a speech recognition unit for recognizing speech using the calculated probabilities of word appearance.
Owner:PANASONIC INTELLECTUAL PROPERTY CORP OF AMERICA
Methods for selecting and controlling devices
ActiveUS20110234366A1Shorter selecting and controlling delayReduce signalingTransmission systemsAssess restrictionWireless controlLighting system
A method of selecting and controlling devices based on wireless communication technology. The wireless controller sends a probe message to one or more devices; each device receives the probe message, obtains information in respect of its relative position with respect to the wireless controller, determines a response time to respond according to a first predefined rule, based on its relative position information: detects response signals from other devices before expiration of the response time; decides whether to send or not to send its response signal according to a second predefined rule and the detecting procedure of response signals from other devices; the wireless controller receives response signals sent by the devices after the comparison of the relative position information of each device with respect to the wireless controller, and selects the target devices from the devices. Embodiments of the present invention reduce the complexity, delay and energy consumption of the selection for wireless devices, and are especially applicable for wireless lighting systems.
Owner:SIGNIFY HLDG BV
Aspherical type imaging lens array
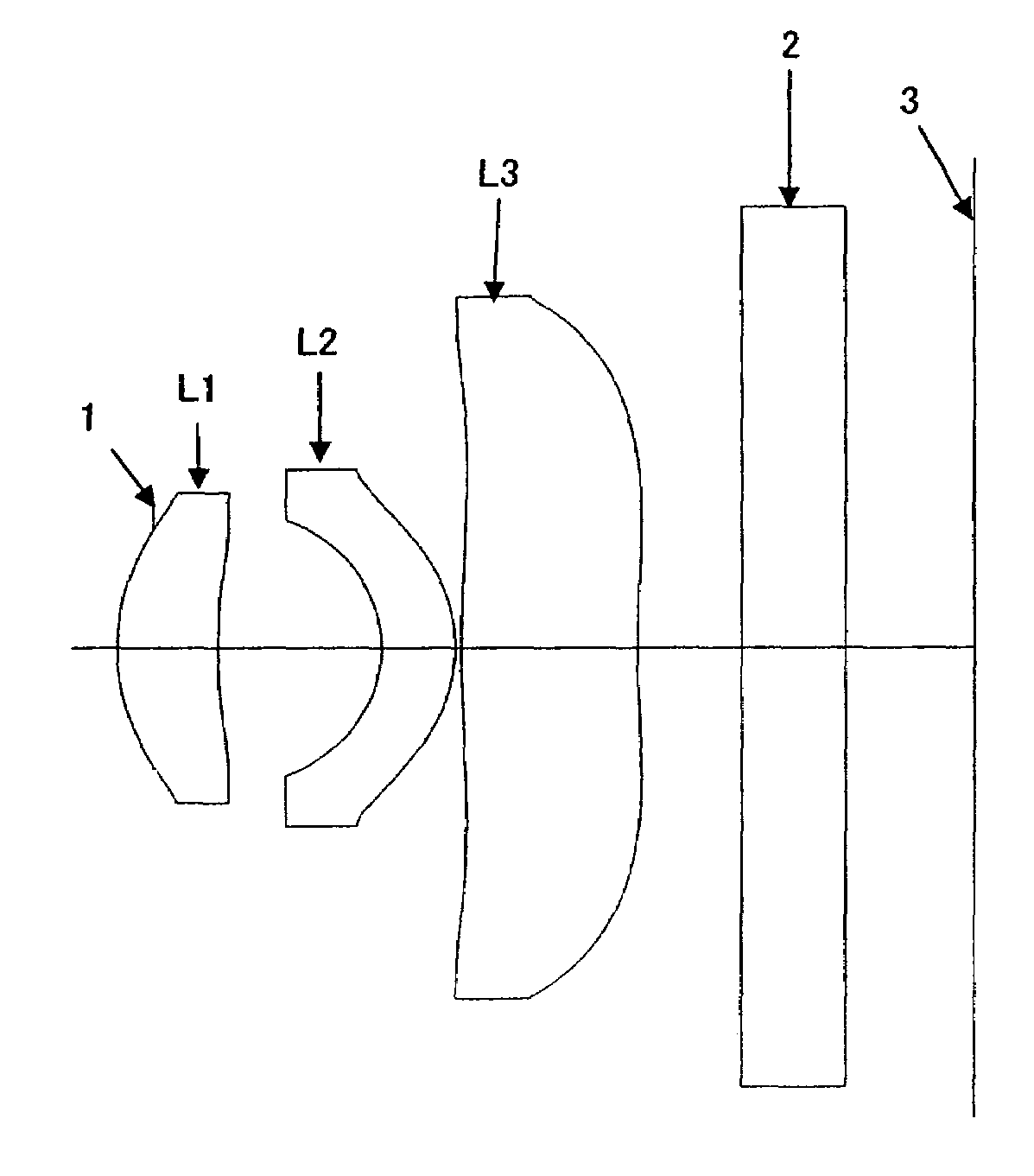
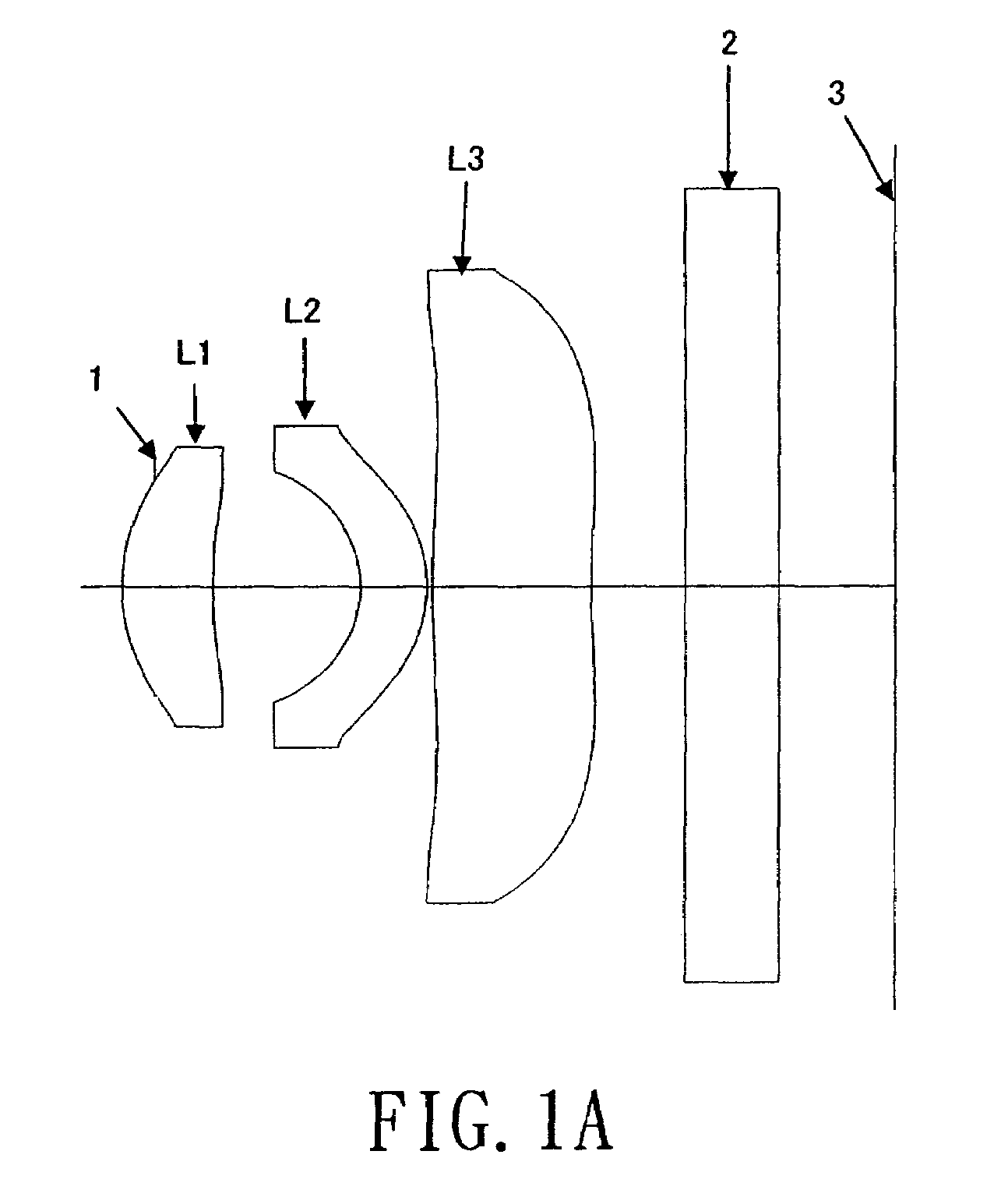
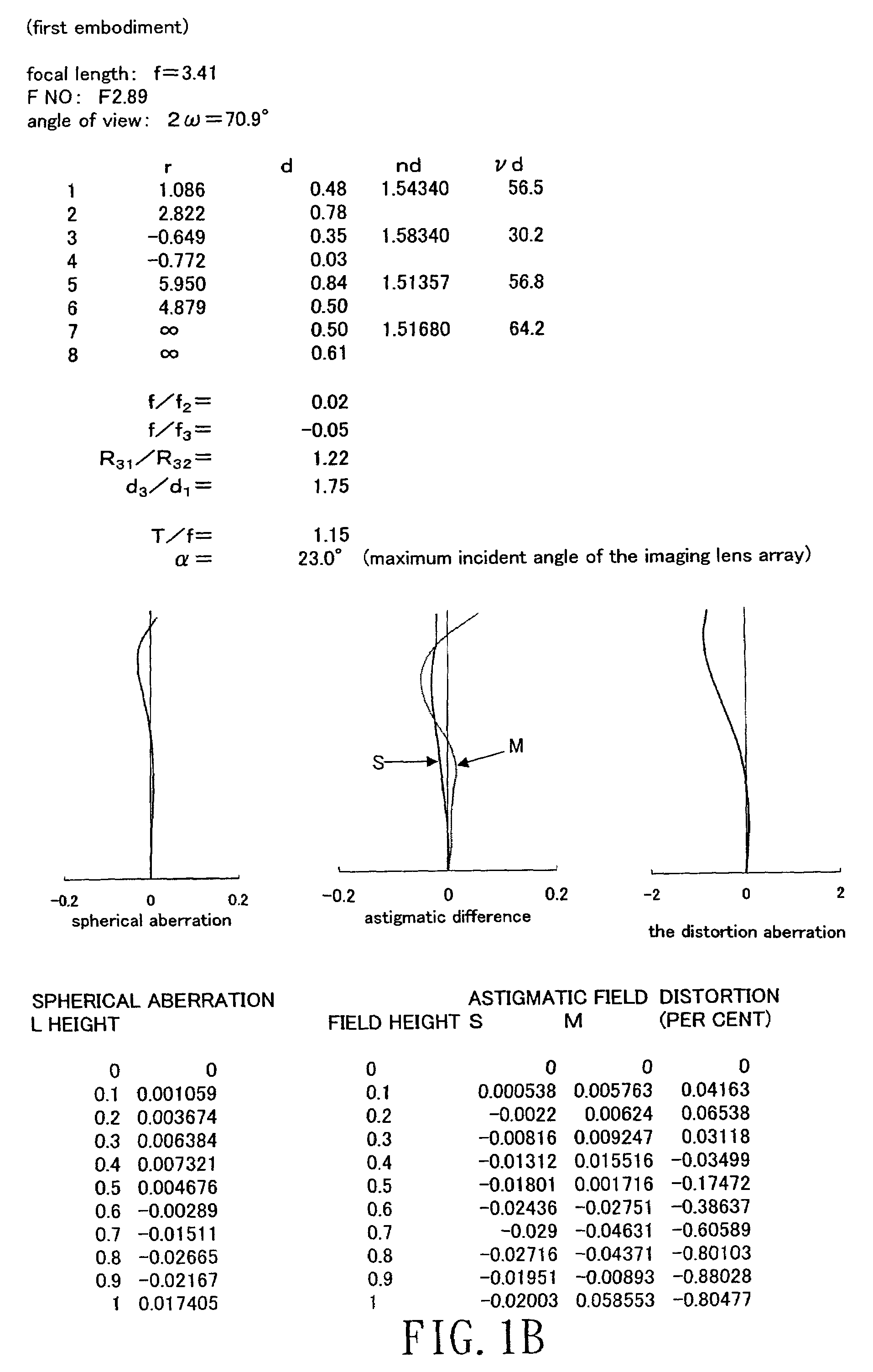
ActiveUS7184225B1Process is complicatedIncrease in overall optical lengthOptical elementsPhysicsMeniscus
An aspherical type imaging lens array comprises, from object side to image side, at least an aperture, a first positive meniscus lens with a convex surface facing the object side, a second meniscus lens with a convex surface facing the image side, and a third lens meniscus lens with a convex surface facing the object side. The respective lenses are made of plastic and has two aspherical surfaces, a focal length of the aspherical type imaging lens array is f, a focal length of the second lens is f2, and a focal length of the third lens is f3, they satisfy the equation: −0.20<f / f2<0.15, and −0.25<f / f3<0.20.
Owner:LARGAN PRECISION
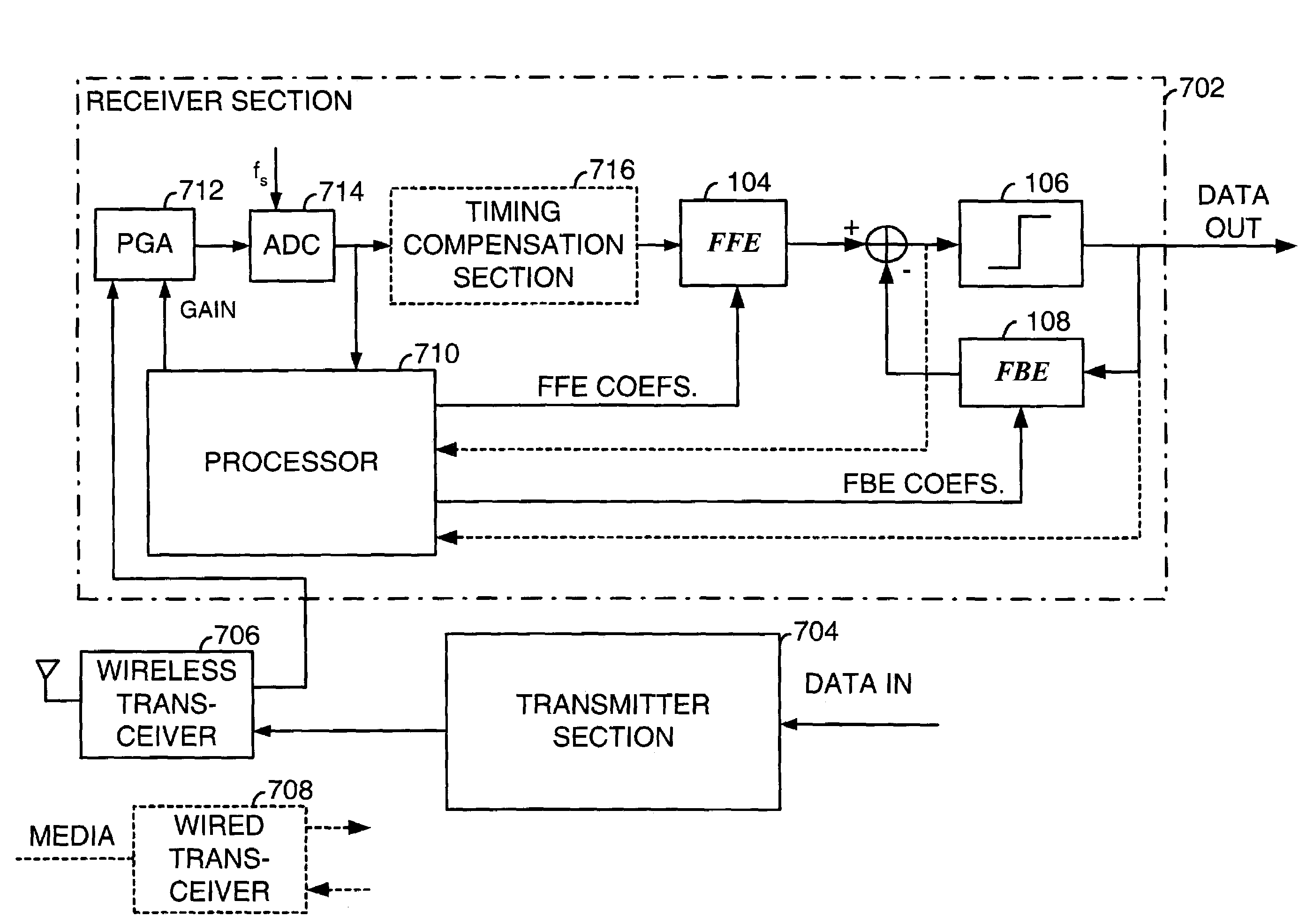
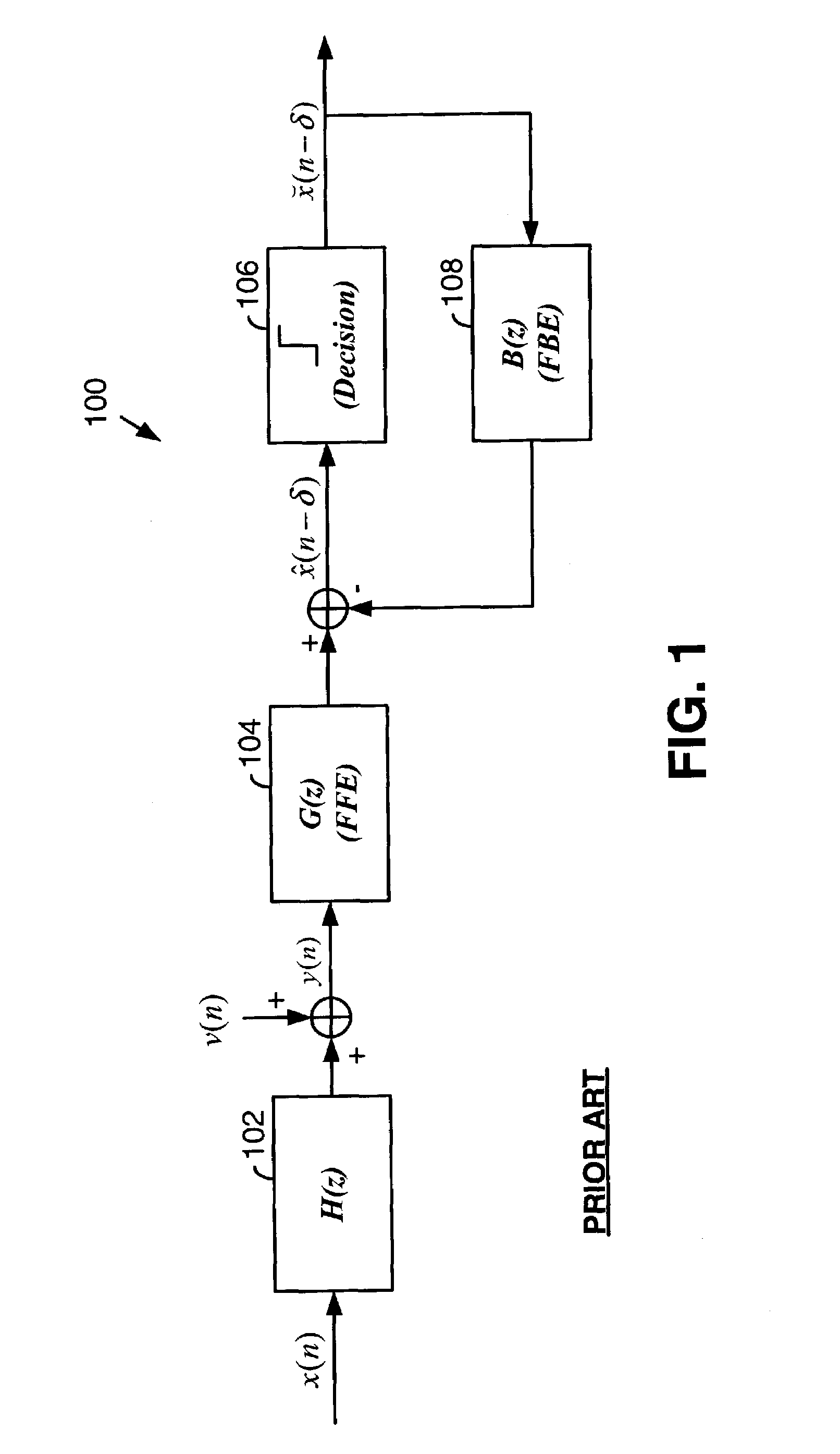
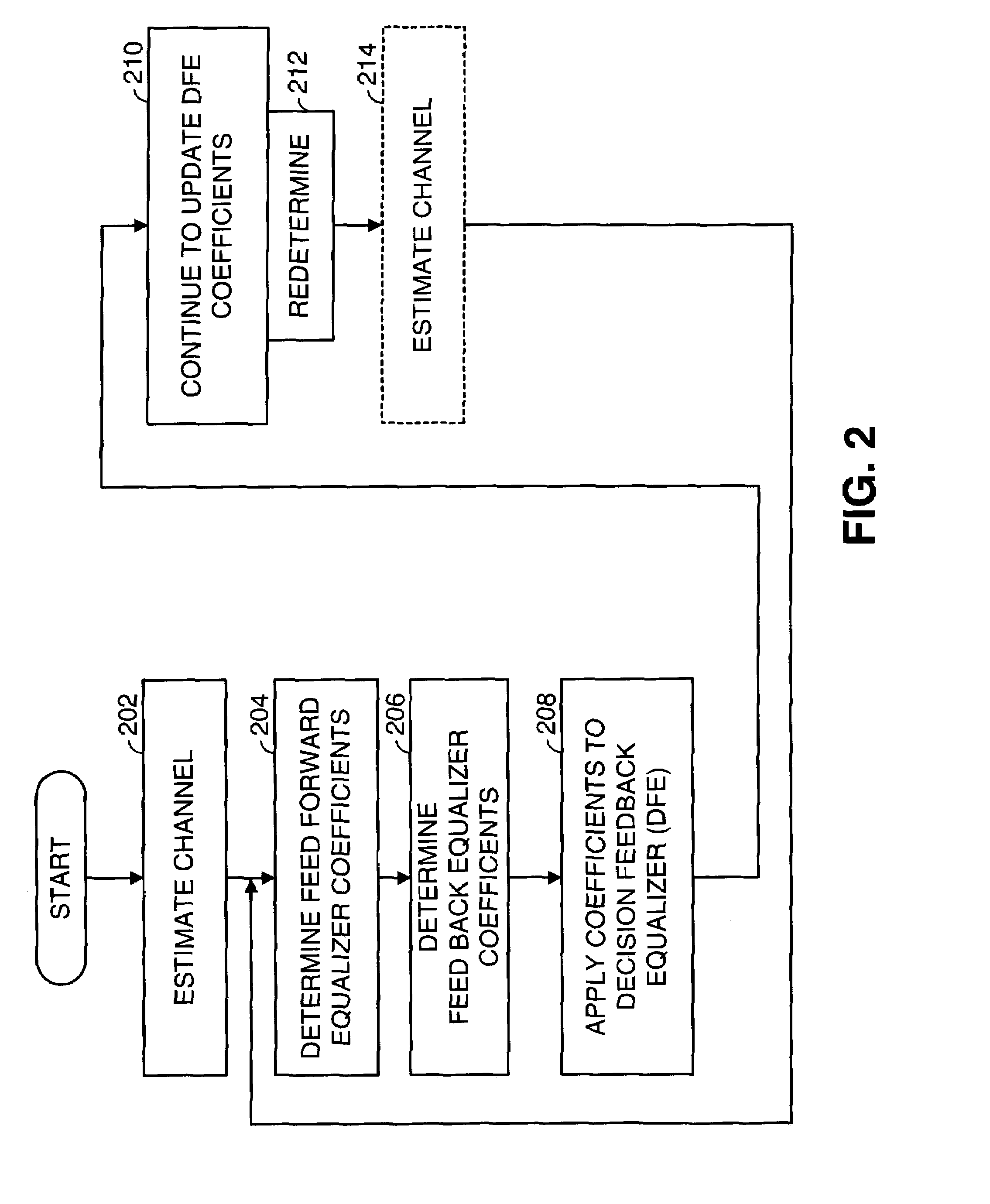
Fast computation of coefficients for a variable delay decision feedback equalizer
InactiveUS7263123B2Reduce computational complexityIncrease computing speedMultiple-port networksDelay line applicationsOptimal decisionComputational problem
Optimal Decision Feedback Equalizer (DFE) coefficients are determined from a channel estimate by casting the DFE coefficient problem as a standard recursive least squares (RLS) problem and solving the RLS problem. In one embodiment, a fast recursive method, e.g., fast transversal filter (FTF) technique, is used to compute the Kalman gain of the RLS problem, which is then directly used to compute MIMO Feed Forward Equalizer (FFE) coefficients. The FBE coefficients are computed by convolving the FFE coefficients with the channel impulse response. Complexity of a conventional FTF algorithm may be reduced to one third of its original complexity by selecting a DFE delay to force the FTF algorithm to use a lower triangular matrix. The length of the DFE may be selected to minimize the tap energy in the FBE coefficients or to ensure that the tap energy in the FBE coefficients meets a threshold.
Owner:AVAGO TECH INT SALES PTE LTD
Method of creating and utilizing diversity in a multiple carrier communciation system
ActiveUS20080182580A1Reduce quality problemsReduce and minimize effectPower managementEnergy efficient ICTFrequency spectrumCarrier signal
In many cellular systems, reusing spectrum bandwidth, creates problems in boundary regions between the cells and sectors where the signal strength received from adjacent base stations or adjacent sector transmissions of a single base station may be nearly equivalent. The invention creates a new type of diversity, referred to as multiple carrier diversity by utilizing multiple carriers, assigning different power levels to each carrier frequency at each base station, and / or offsetting sector antennas. The cell and / or sector coverage areas can be set so as to minimize or eliminate overlap between cell and / or sector boundary regions of different carrier frequencies. Mobile nodes traveling throughout the system can exploit multiple carrier diversity by detecting carriers and selecting to use a non-boundary carrier based on other system criteria in order to improve performance. Boundary carriers may, but need not be, identified and excluded from consideration for use by a wireless terminal.
Owner:QUALCOMM INC
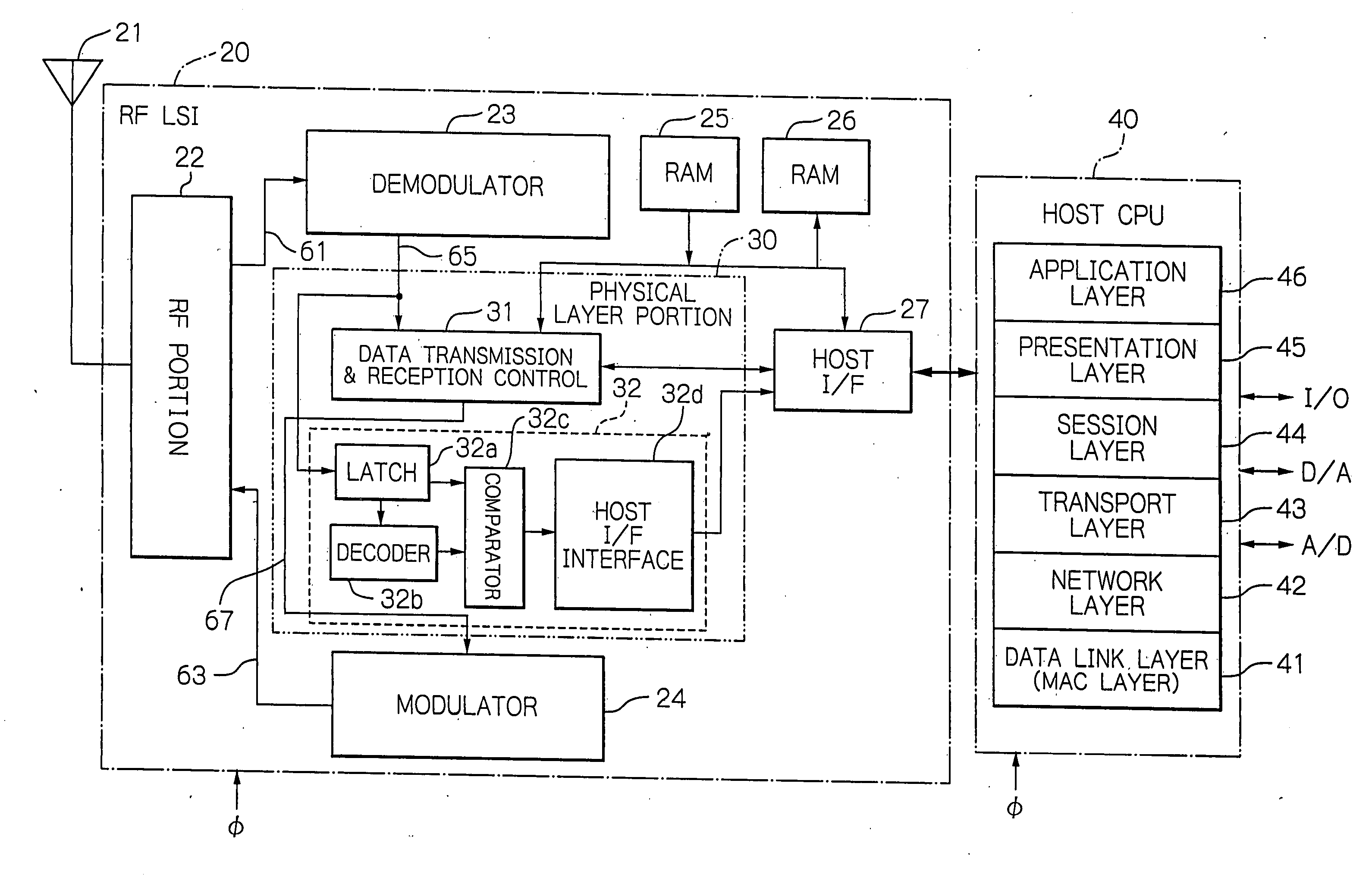
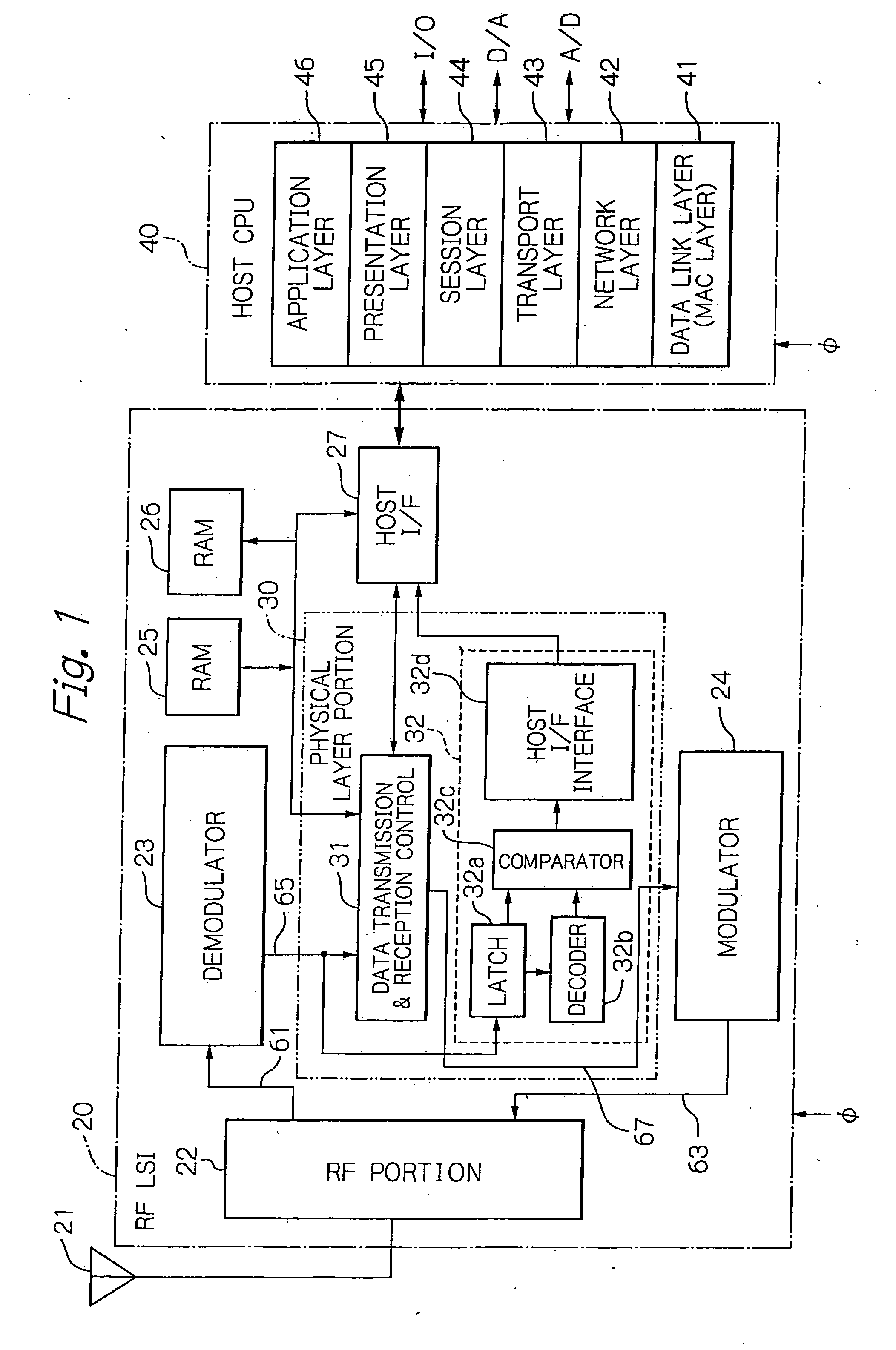
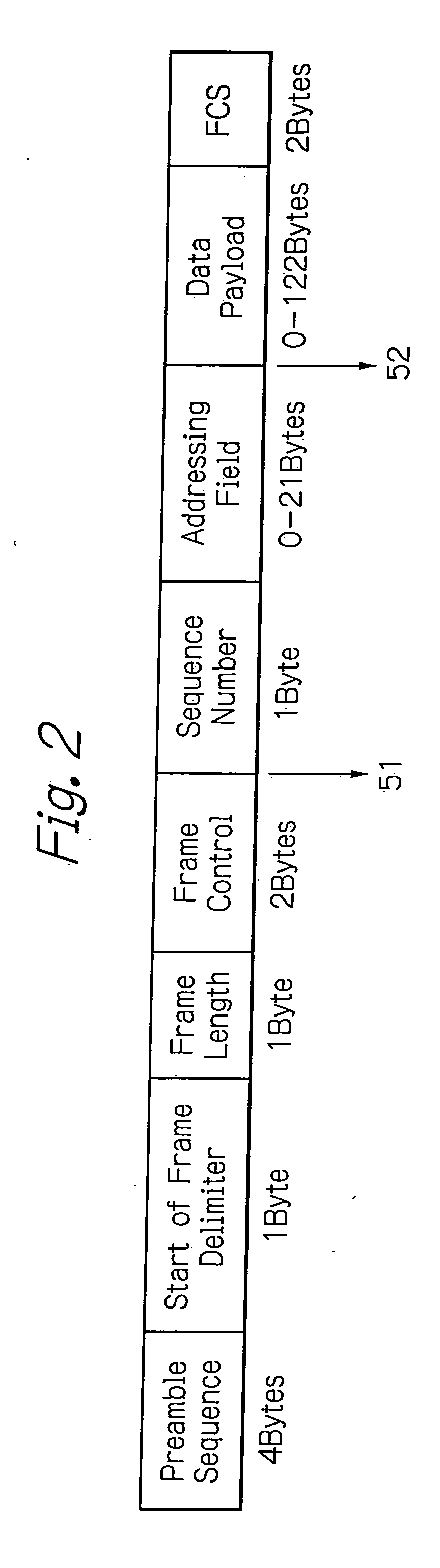
Radio frequency integrated circuit having a physical layer portion integrated therein
InactiveUS20060186973A1Many timesLower performance requirementsData switching by path configurationCoupling devicesPhysical layerTransfer mode
A ZigBee-compliant radio frequency LSI includes a physical layer portion and a modulator. The physical layer portion has an RF portion, a demodulator, a data transmission and reception control, and a transfer mode determination portion. The transmission and reception control converts, during reception, symbol data received by the demodulator into the byte data received, and outputs, during transmission, the symbol data to be transmitted to the modulator. The determination portion determines, when the first identification data in the received data from the RF portion necessary for determining the received data transfer mode are fixed, the data length of the subsequent second identification data. The determination portion latches, when data corresponding to the determined length of the second identification data are fixed, the data necessary for determining the received data transfer mode to transfer the data to the MAC layer.
Owner:LAPIS SEMICON CO LTD
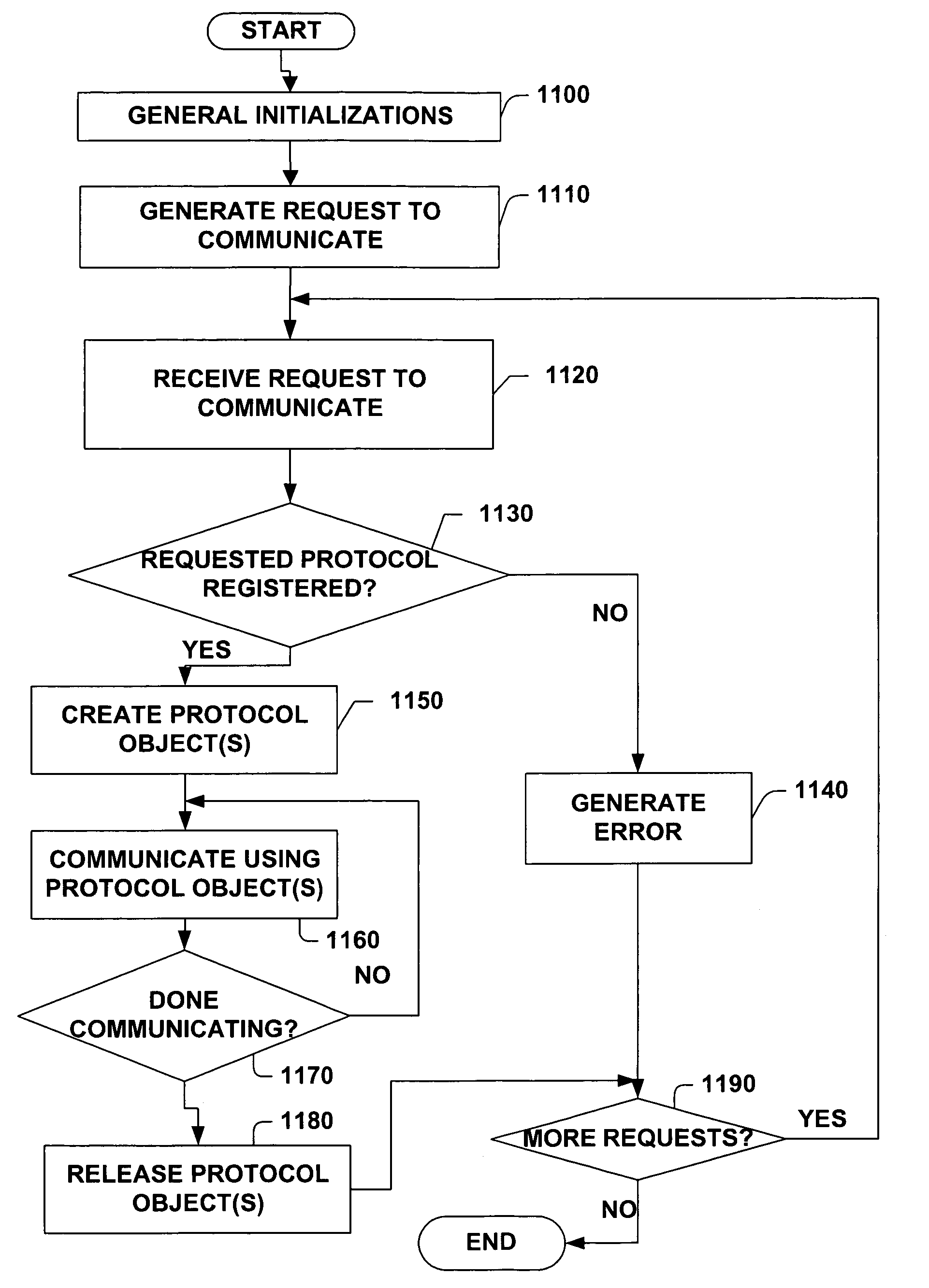
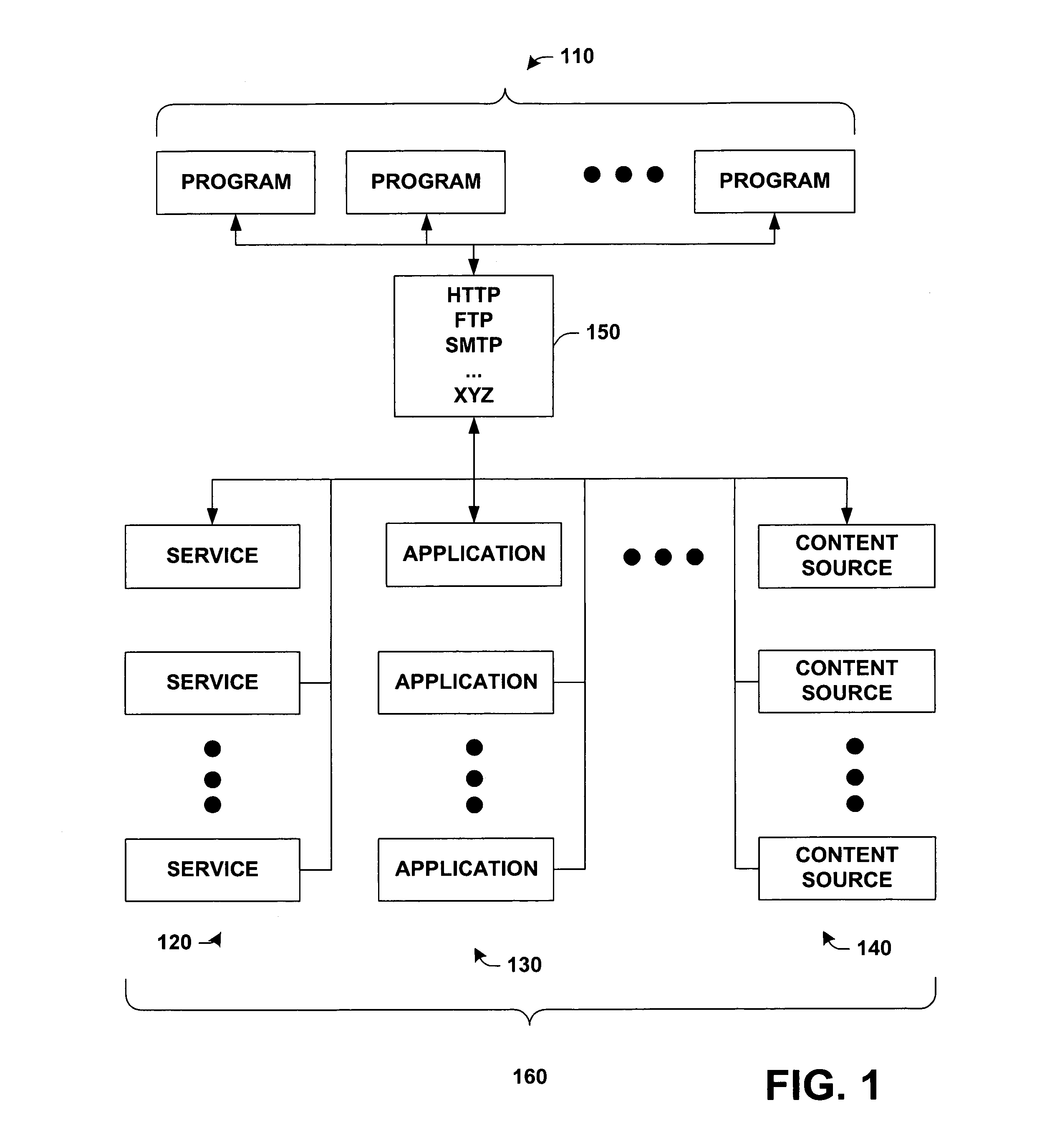
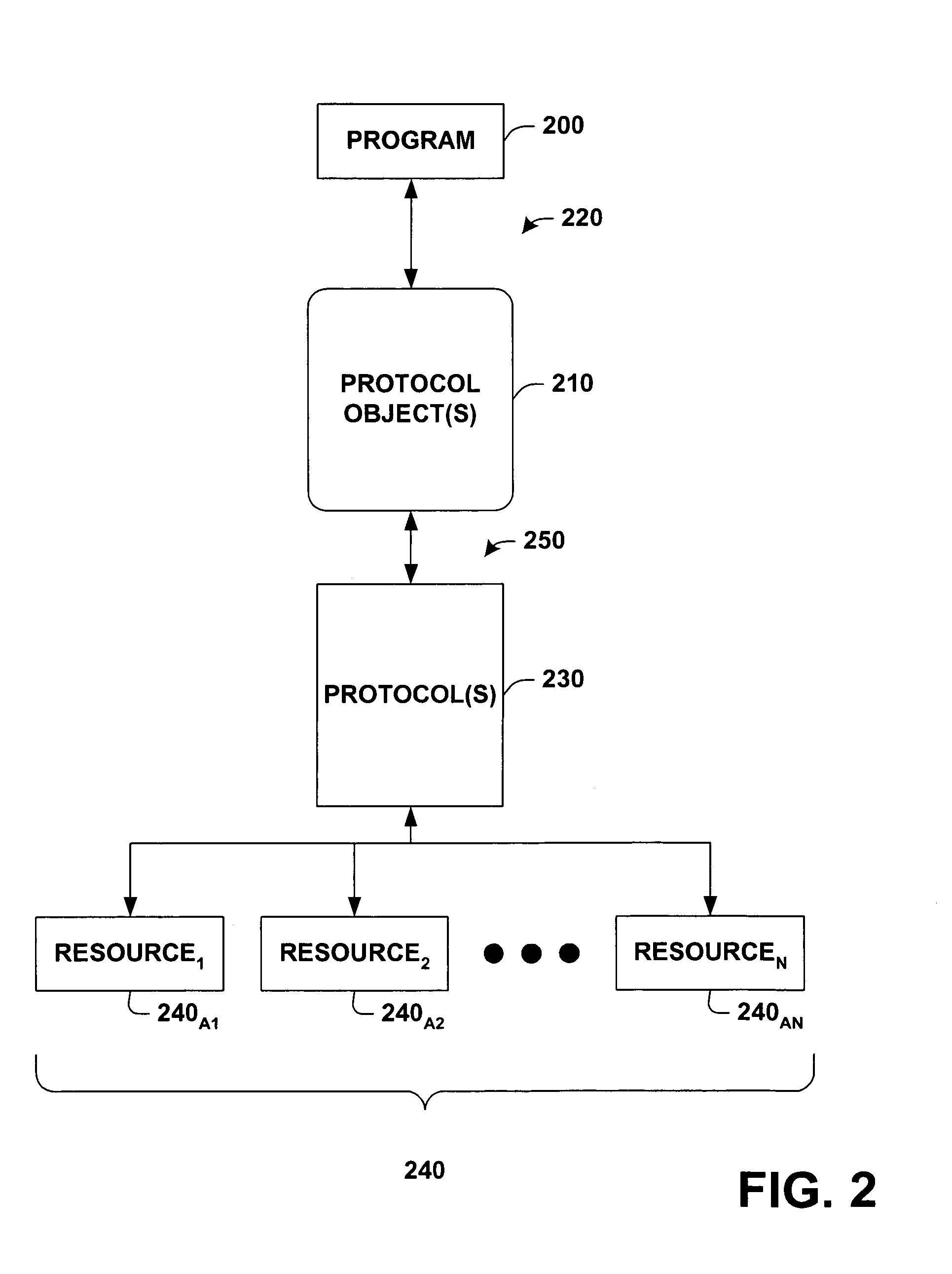
Protocol agnostic request response pattern
InactiveUS6996833B1Enhanced interactionFacilitates receiving and sending messageMultiprogramming arrangementsMultiple digital computer combinationsNetworking protocolProtocol processing
A system and method for facilitating communications over a protocol is provided. The system includes a class factory that holds identifiers associated with determining which, if any, registered protocol object creator should be employed to create a protocol object. The protocol object implements an abstract network protocol base class. The protocol object is employed to abstract details of communicating over a protocol and to provide a byte stream interface to communications occurring over the protocol, while removing protocol specific code from an application program. The method includes creating an instance of a protocol object from a source of registered protocol handlers based on a request to communicate over a protocol and using a base class API to communicate over the protocol through the protocol object.
Owner:MICROSOFT TECH LICENSING LLC
Liquid transfer device
InactiveUS20090074624A1Potential riskEasy to controlAnalysis using chemical indicatorsAnalysis by subjecting material to chemical reactionInterior spaceTest agent
A liquid transfer device is provided for biochemical assay, including a pipette forming an interior space extending in an axial direction and having first and second ends. The first end forms an opening and the second end forms an enclosed variable volume, whereby variation of the volume causes a change of pressure to selectively induce a suction force and a releasing force in the pipette. An analysis container includes a plurality of receptacles retained by a slab. A film covers the slab to seal the container. A movement control device includes a manipulator that releasably holds the pipette and a tray that forms a cavity for receiving and retaining the container. The pipette and the tray are movable with respect to each other in order to fill / draw test agent into / out of the receptacles.
Owner:LIANG DON
Nonvolatile semiconductor storage and its manufacturing method
InactiveUS20050079662A1Complicated processEasy to integrateTransistorSolid-state devicesSilicon dioxideSemiconductor
To achieve a higher operating speed, higher reliability, and lower power consumption by reducing the thickness of an inter-poly silicon insulator film between a floating gate and a control gate of a flash memory, a silicon dioxide film, a silicon nitride film, tantalum pentoxide, and a silicon dioxide film are formed in a multilayer structure to serve as the inter-poly insulator film between a floating gate and a control gate. With this configuration, tantalum pentoxide formed on the silicon nitride film has a dielectric constant of 50 or more, which is higher than that of the silicon dioxide film, and the thickness of the inter-poly silicon insulator film can be reduced.
Owner:RENESAS ELECTRONICS CORP
Injection systems for delivery of fluids to joints
InactiveUS8002736B2Precise deliveryPatient benefitUltrasonic/sonic/infrasonic diagnosticsAutomatic syringesAnatomical structuresCatheter
Systems for injecting fluids and / or other materials into a targeted anatomical location, in particular, an intra-articular space, include a handpiece assembly having a proximal end and a distal end, a needle extending from the distal end of the handpiece assembly, a fluid delivery module comprising a cassette and a fluid transfer device. A conduit is generally configured to place the fluid delivery module in fluid communication with the handpiece assembly. Medications, formulations and / or other fluids or materials contained within vials that are secured to the fluid delivery module can be selectively delivered into an anatomy through a needle located at the distal end of the handpiece assembly. In some embodiments, ultrasound or other imaging technologies can be used to locate a joint or other targeted anatomical location.
Owner:CARTICEPT MEDICAL
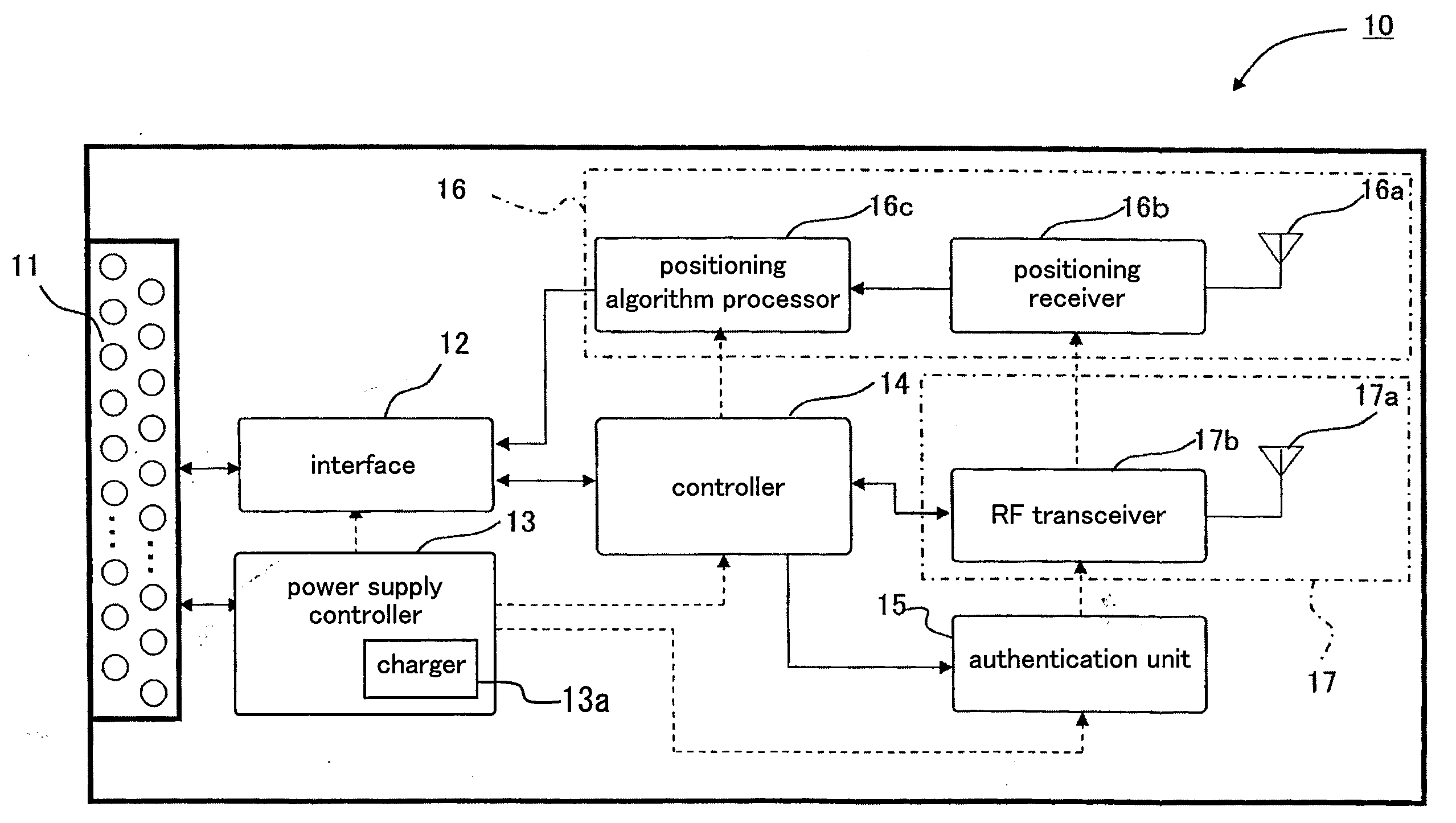
Modem card
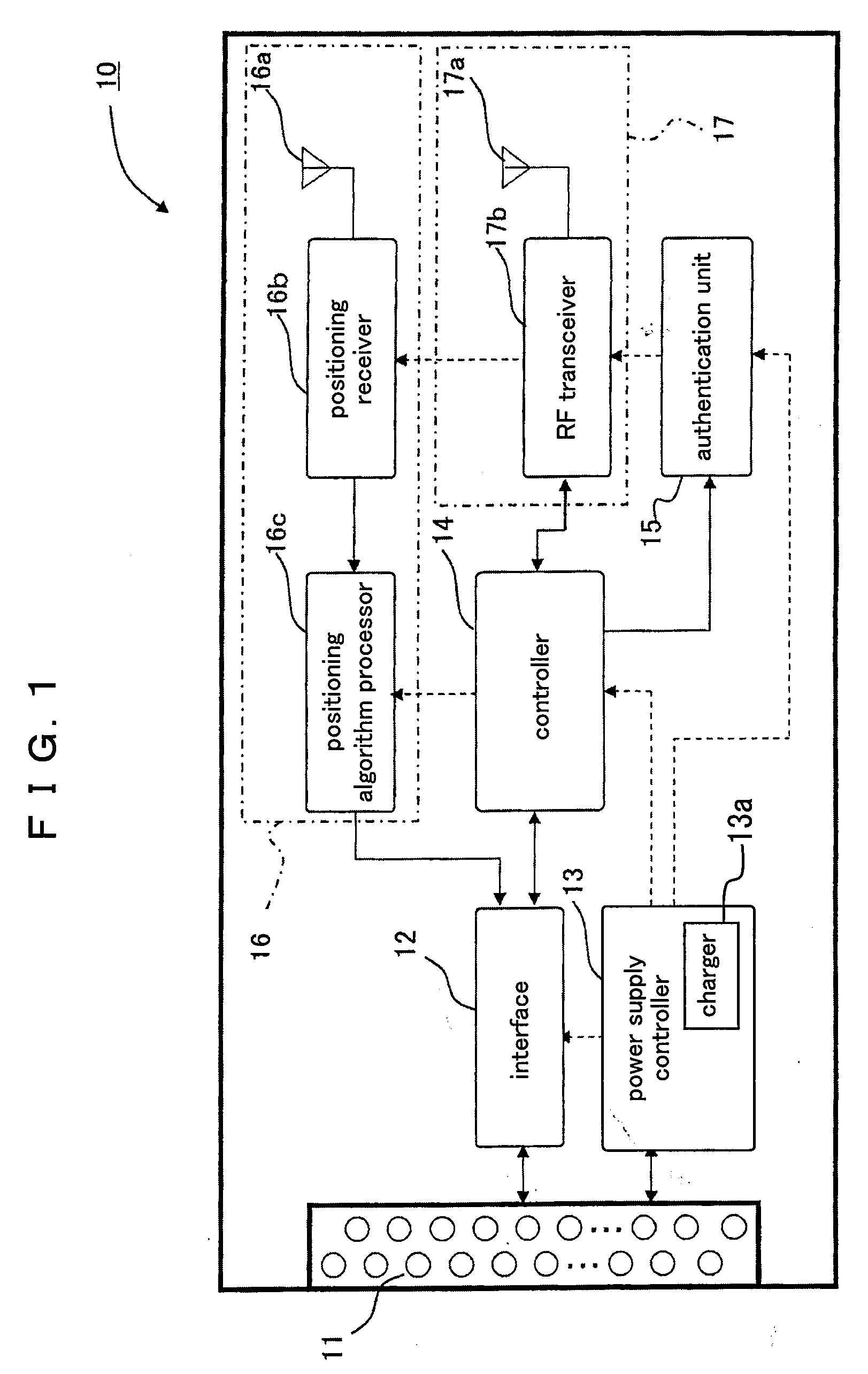
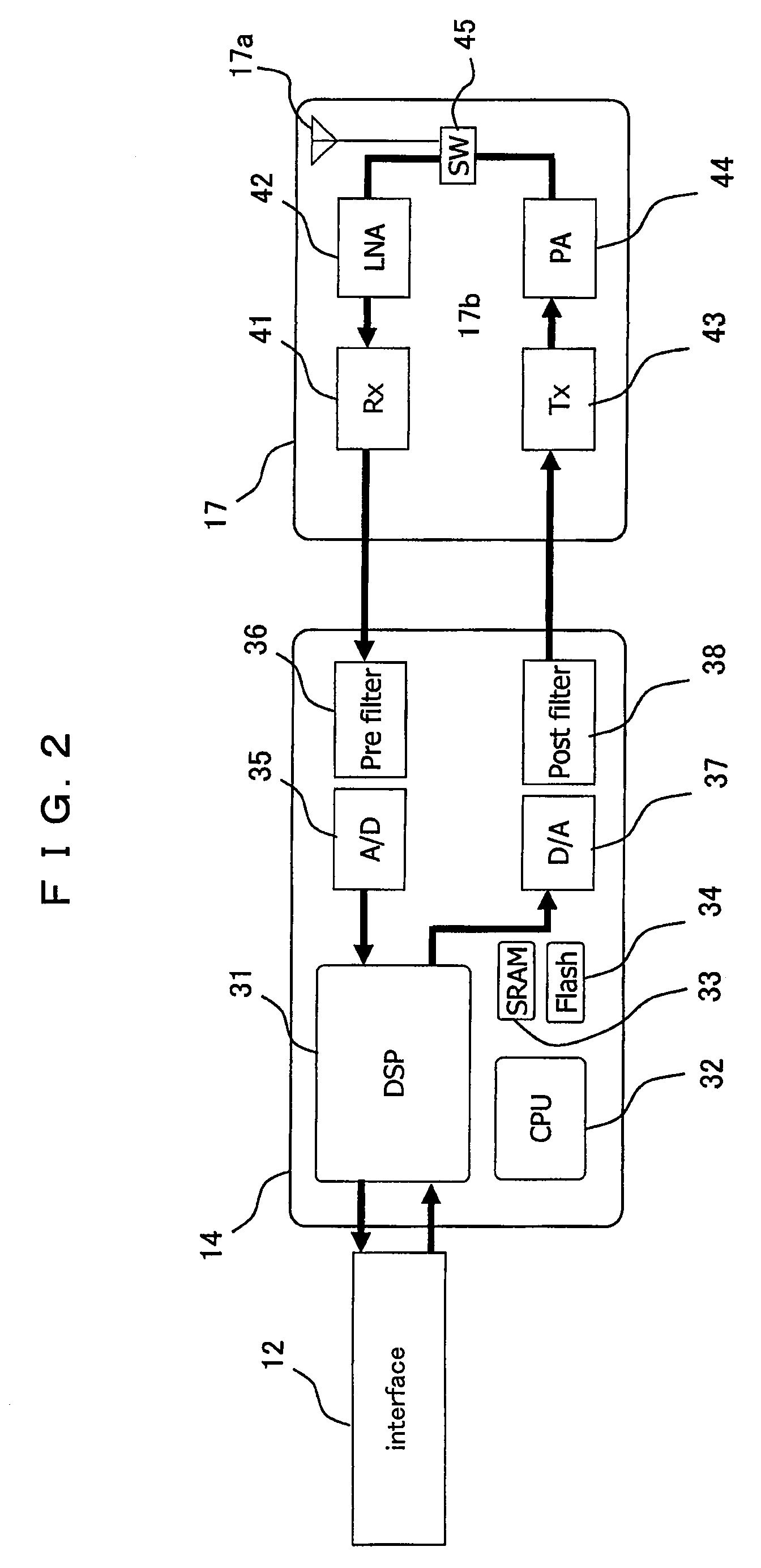
InactiveUS20090270127A1Easy to manageIncrease in cost and sizeDigital data processing detailsSubstation equipmentCommunication unitCommunication device
A communication unit communicates with an external communication device. An authentication unit authenticates a subscription right for communication. A position detector detects information of a current position. An interface carries out, according to a standardized signal mode, reception and transmission of input and output signals between an application device selected as a communication terminal from a group of application devices and the communication unit and the position detector.
Owner:PANASONIC CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com