Synthesis of poly-(p-aryleneethynylene)s in neat water under aerobic condit
a technology of arylene and ethylene, which is applied in the field of polymer synthesis, can solve the problems of inability to implement in the presence of water, inability to synthesize carbyne precursors for adimet protocols, and inability to achieve inert reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
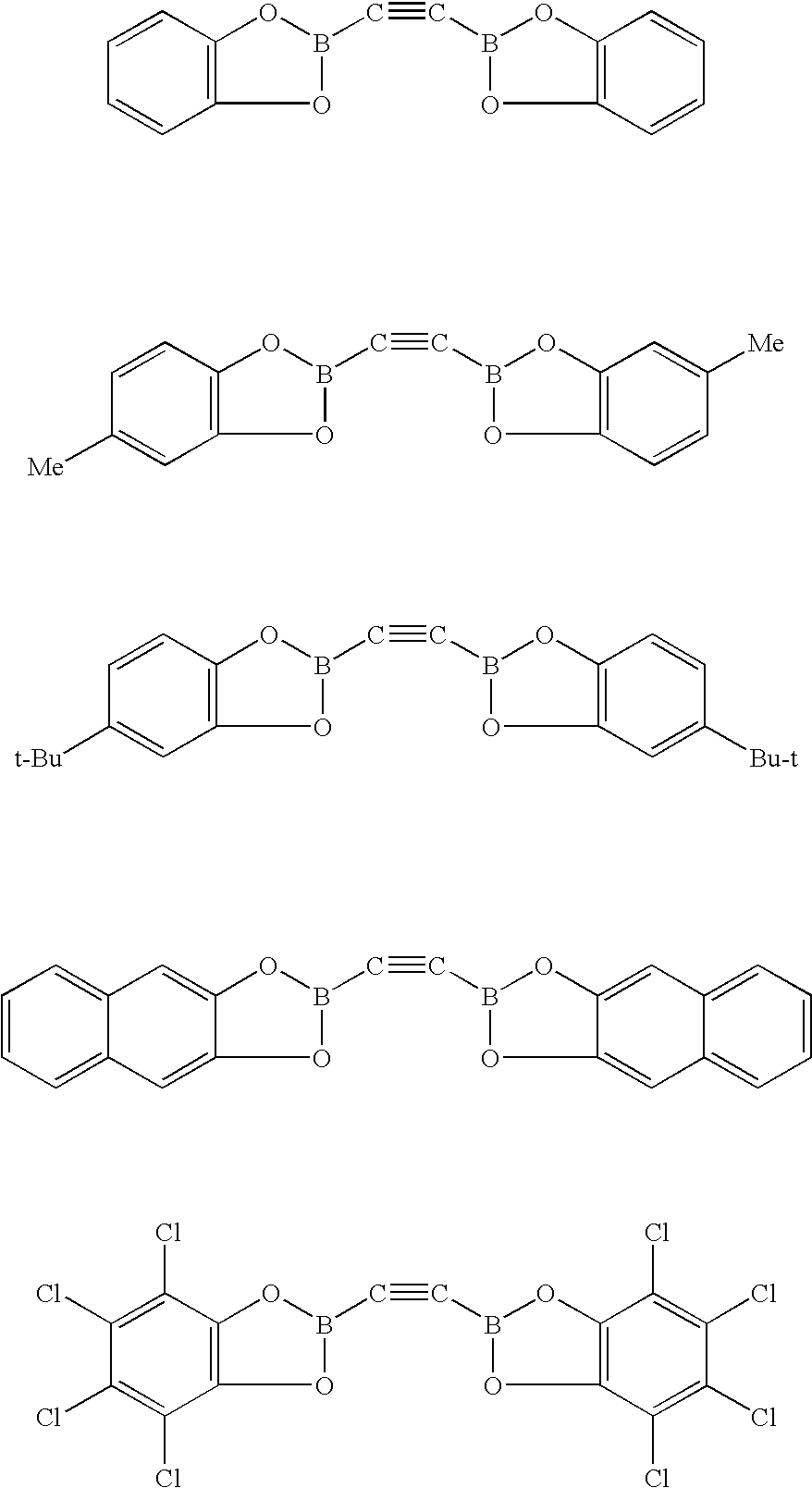
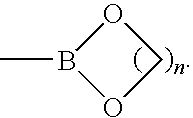
[0100]Certain reactions involving alkynylboranes, alkynylboronic acid or alkynylborate derivatives, and alkynyltrifluoroborates are accepted. Reactions involving reagents that possess boron functionality at the 1- and 2-carbon positions of ethyne, however, have not yet been reported. This motivated the synthesis of [1,2-bis(4′,4′,5′,5′-tetramethyl[1′,3′,2′]dioxaborolan-2′-yl)ethyne (B2C2). The 1H NMR spectrum of B2C2 is shown in FIG. 1. FIG. 2 depicts the 13C NMR spectrum of B2C2 in C6D6, and FIG. 3 depicts the 11B NMR spectrum of B2C2 in C6D6.
[0101]Reaction of dilithioacetylide with 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, followed by treatment with anhydrous HCl, cleanly afforded B2C2 in high yield (Scheme 1). B2C2 was recrystallized from hexanes, giving a robust crystalline solid (mp=270° C.) that is stable under ambient atmosphere for at least 1 year. B2C2 exhibited high solubility in basic water and virtually all organic solvents.
[0102]X-ray quality crystals of B2C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com