Universal method for constructing chiral organic molecular cage
A molecular cage and universal technology, applied in the field of synthesis of chiral porous materials, can solve the problems of expensive reaction raw materials and difficulty in mass production, and achieve a wide range of ligands, simple and efficient ligand design, and reaction easy to scale up effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
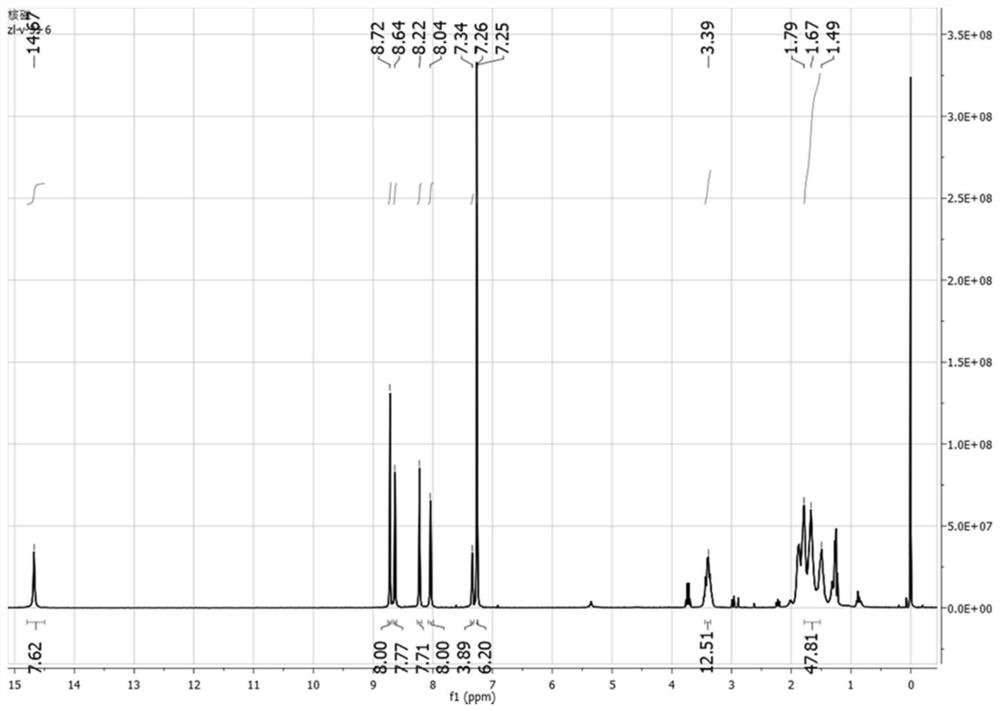
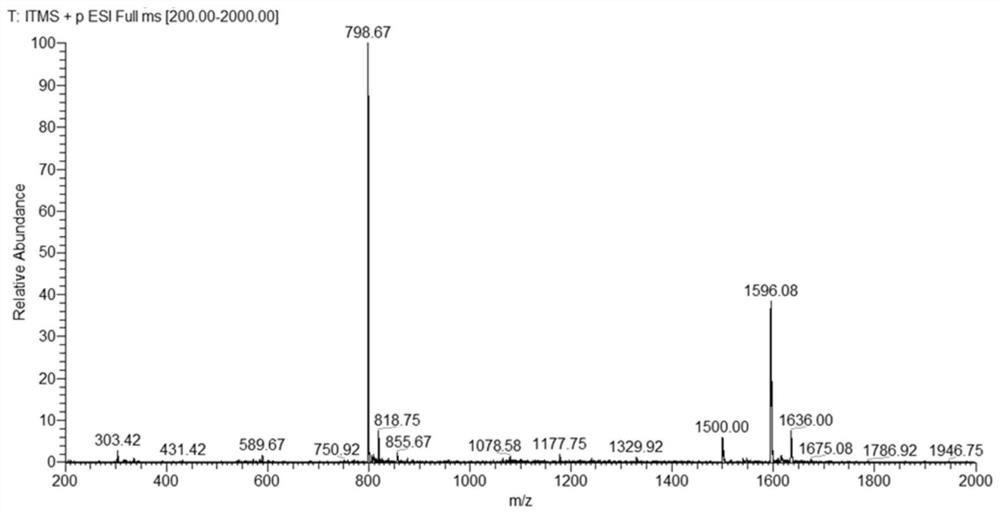
[0032] Example 1 Synthesis of V-type pyridine[3+6]-type chiral molecular cage
[0033] The reaction formula is as follows:
[0034]
[0035] Synthesis of V-type pyridine tetraaldehyde
[0036] Weigh 3,5-dibromopyridine (4.68 g, 20 mmol), p-methoxyphenylboronic acid (6.68 g, 44 mmol), cesium carbonate (9.75 g, 30 mmol), tetrakis(triphenylphosphine) palladium (0.05g, 0.04 mmol), put into a 150ml two-necked bottle, add 80 ml of toluene, 20 ml of deionized water, reflux at 100°C under the protection of inert gas, react for 24 hours and cool to room temperature, a large number of transparent crystals precipitated in the solution, filtered and washed with water Compound 1 (4.2g, 72%) was obtained; 4.2 g of compound 1 and 20 g of pyridine hydrochloride were weighed into a 100 ml single-port reaction flask, refluxed at 190°C under nitrogen protection for 5 h, cooled to room temperature, and 80 ml of deionized After stirring water at room temperature for 0.5 h, a large amount of w...
Embodiment 2
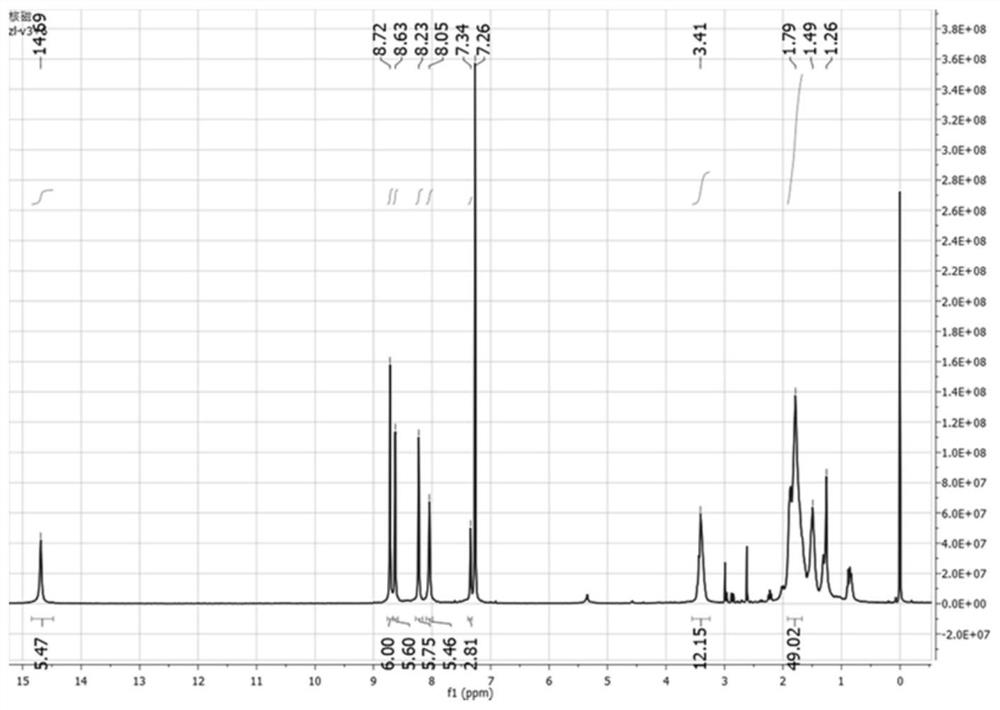
[0039] Example 2 Synthesis of linear naphthyl [3+6] chiral molecular cage
[0040] The reaction formula is as follows:
[0041]
[0042] Synthesis of linear naphthyl tetraaldehyde:
[0043]Weigh 1,4-dibromonaphthalene (5.66 g, 20 mmol), p-methoxyphenylboronic acid (6.68 g, 44 mmol), cesium carbonate (9.75 g, 30 mmol), tetrakis(triphenylphosphine) Add palladium (0.05 g, 0.04 mmol) to a 150 ml two-necked bottle, add 80 ml of toluene, 20 ml of deionized water, reflux at 100 ° C under the protection of an inert gas, react for 24 h and cool to room temperature, a large number of white needle-like crystals precipitate in the solution, filter Wash with water to obtain compound 4 (6.10 g, 89%); weigh 6 g of compound 4 and 20 g of pyridine hydrochloride into a 100 ml single-port reaction flask, reflux at 190°C under nitrogen protection for 5 h, cool to room temperature, and add 80 ml to Ionized water was stirred at room temperature for 0.5 h, and a large amount of white powder was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com