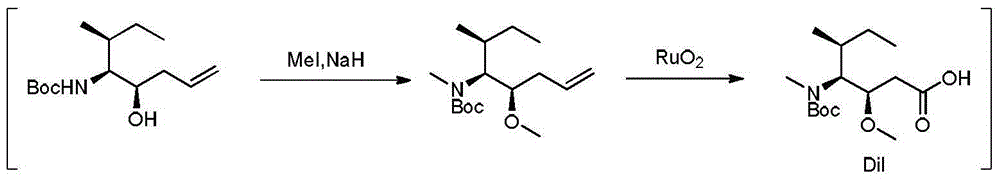
Preparation method of tert-butyl (3R, 4S, 5S)-5-hydroxy-3-methyl-7-ocentyl-4-carbamate
A carbamate, tert-butyl technology, applied in the field of intermediate synthesis, can solve the problems of low stereoselectivity, inconvenient operation, low reaction yield, etc., to simplify the experimental operation process, reduce the reaction time, The effect of cheap and easy to obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
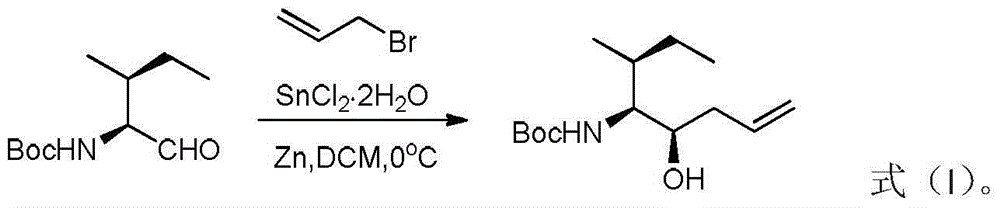
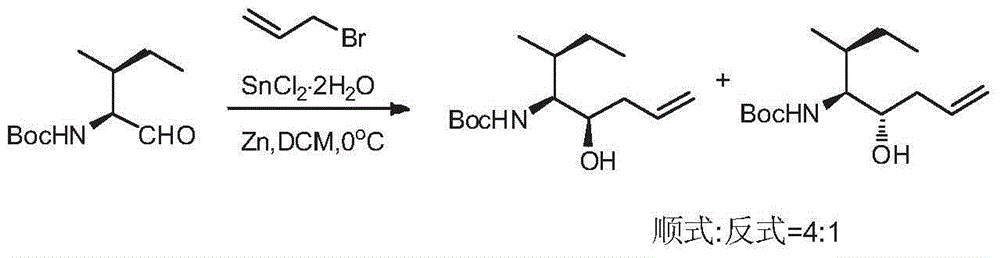
[0024] At room temperature, measure N-tert-butoxycarbonyl-L-isoleucinaldehyde (3.22g, 15mmol), allyl bromide (3.64g, 30mmol), dissolve in dichloromethane (35mL) under uniform stirring, Cool in an ice-water bath to 0°C, then add stannous dichloride dihydrate (6.78g, 30mmol) and zinc powder (1.96g, 30mmol) respectively. After the addition, keep the ice-water bath, stir and react for 0.5 hours, and monitor by TLC to determine the end point of the reaction. After the reaction was completed, the ice-water bath was removed, the solid in the reaction flask was removed by filtration, the filtrate was concentrated under reduced pressure, and the residue was directly separated and purified by column chromatography, and the eluent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 5:1. , 2.73 g of cis-tert-butyl (3R, 4S, 5S)-5-hydroxy-3-methyl-7-octene-4-carbamate was obtained, and the yield was 71%.
[0025] 1 HNMR (400MHz, CDCl 3 )δ5.80-5.90(m,1H), 4.98-5...
Embodiment 2
[0027] At room temperature, measure N-tert-butoxycarbonyl-L-isoleucinaldehyde (3.22g, 15mmol), allyl bromide (3.28g, 27mmol), dissolve in dichloromethane (32mL) under uniform stirring, Cool in an ice-water bath to 0°C, then add stannous dichloride dihydrate (6.10g, 27mmol) and zinc powder (1.76g, 27mmol) respectively. After the addition, keep the ice-water bath, stir and react for 0.5 hours, and monitor by TLC to determine the end point of the reaction. After the reaction was completed, the ice-water bath was removed, the solid in the reaction flask was removed by filtration, the filtrate was concentrated under reduced pressure, and the residue was directly separated and purified by column chromatography, and the eluent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 5:1. , 2.65 g of cis-tert-butyl (3R, 4S, 5S)-5-hydroxy-3-methyl-7-octene-4-carbamate was obtained, and the yield was 69%.
[0028] 1 HNMR (400MHz, CDCl 3 )δ5.80-5.90(m,1H), 4.98-5...
Embodiment 3
[0030] At room temperature, measure N-tert-butoxycarbonyl-L-isoleucinaldehyde (3.22g, 15mmol), allyl bromide (4.0g, 33mmol), dissolve in dichloromethane (35mL) under uniform stirring, Cool in an ice-water bath to 0°C, then add stannous dichloride dihydrate (7.46g, 33mmol) and zinc powder (2.16g, 33mmol) respectively. After the addition, keep the ice-water bath, stir and react for 0.5 hours, and monitor by TLC to determine the end point of the reaction. After the reaction was completed, the ice-water bath was removed, the solid in the reaction flask was removed by filtration, the filtrate was concentrated under reduced pressure, and the residue was directly separated and purified by column chromatography, and the eluent was a mixed solvent of petroleum ether and ethyl acetate with a volume ratio of 5:1. , 2.69 g of cis-tert-butyl (3R, 4S, 5S)-5-hydroxy-3-methyl-7-octene-4-carbamate was obtained, and the yield was 70%.
[0031] 1 HNMR (400MHz, CDCl 3)δ5.80-5.90(m,1H), 4.98-5.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com