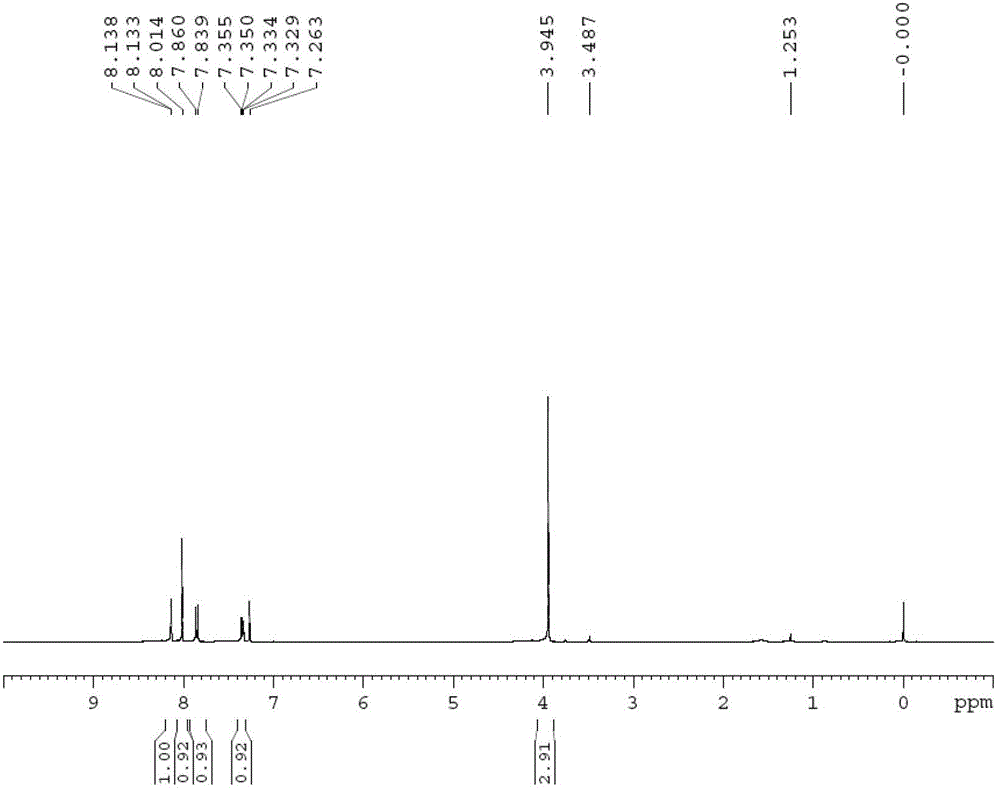
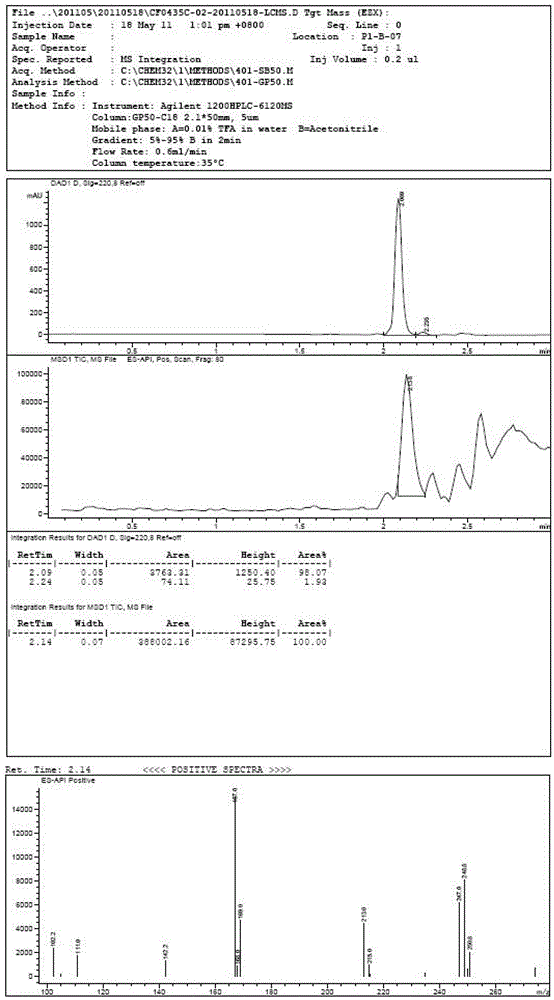
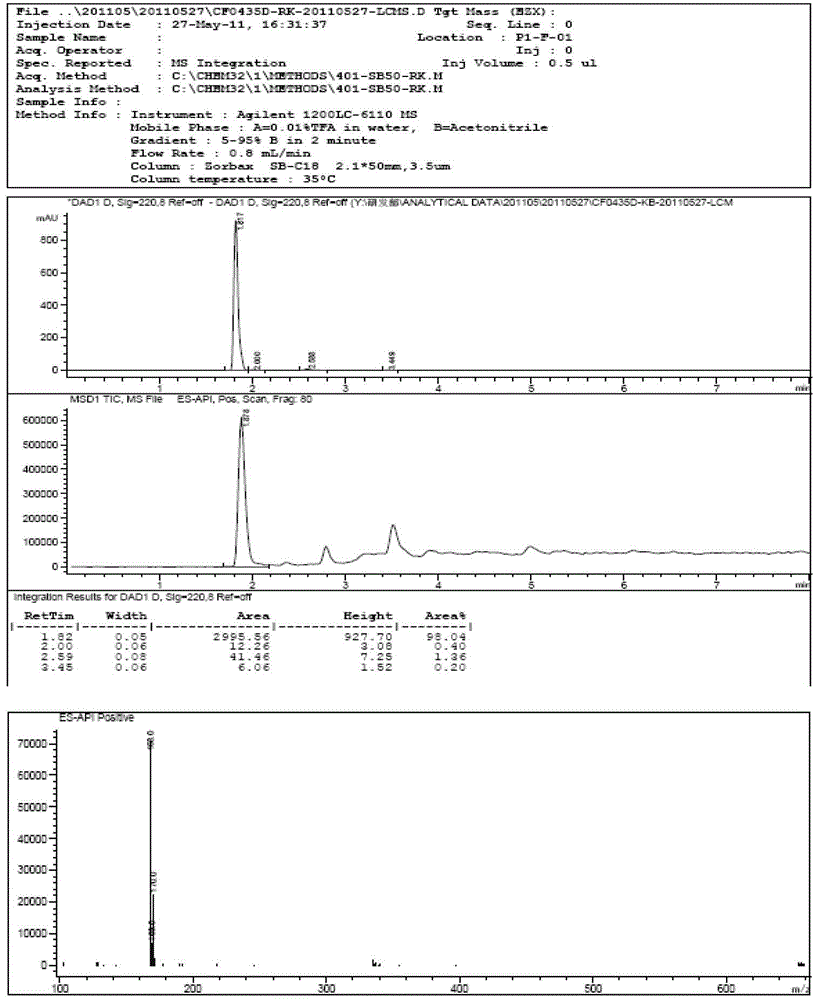
Method for synthesizing 5-isoindolone chloride
The technology of a chloroisoindolinone and a synthetic method is applied in the synthesis field of 5-chloroisoindolinone, can solve the problems such as inability to produce a target compound, a single monobrominated product, no yield, etc., and achieves considerable economic benefits, Easy to scale up production, improve yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A kind of synthetic method of 5-chloroisoindolinone, concrete steps are as follows:
[0048] Step 1: Esterification to obtain methyl 4-chloro-2-methylbenzoate
[0049]15.4 g of 4-chloro-2-methylbenzoic acid, 1.0 ml of sulfuric acid and 200 ml of methanol were heated to reflux overnight. After distilling off the solvent, dilute with 200 ml of ethyl acetate, wash with 1 M, 100 ml of sodium hydroxide solution, dry over anhydrous sodium sulfate and concentrate to obtain 15.5 g of a colorless oily product with a yield of 93%.
[0050] Step 2: Dibromination reaction to give methyl 4-chloro-2-dibromomethylbenzoate
[0051] 15.5 g of 4-chloro-2-dibromomethylbenzoic acid methyl ester obtained in step 1, 30.0 g of NBS, 2.0 g of benzoyl peroxide and 150 ml of carbon tetrachloride were heated to reflux for 6 hours, and the insoluble solids were removed by filtration. The filtrate was washed with saturated sodium carbonate solution, dried with anhydrous sodium sulfate, concentrate...
Embodiment 2
[0057] A kind of synthetic method of 5-chloroisoindolinone, concrete steps are as follows:
[0058] Step 1: Esterification to obtain methyl 4-chloro-2-methylbenzoate
[0059] 15.0 g of 4-chloro-2-methylbenzoic acid, 1.0 ml of sulfuric acid and 180 ml of methanol were heated to reflux overnight. After distilling off the solvent, dilute with 200 ml of ethyl acetate, wash with 1 M, 100 ml of sodium hydroxide solution, dry over anhydrous sodium sulfate and concentrate to obtain 15.1 g of a colorless oily product with a yield of 93%.
[0060] Step 2: Dibromination reaction to give methyl 4-chloro-2-dibromomethylbenzoate
[0061] 15.1 g of 4-chloro-2-dibromomethylbenzoic acid methyl ester obtained in step 1, 29.2 g of NBS, 2.0 g of benzoyl peroxide and 150 ml of carbon tetrachloride were heated to reflux for 6 hours, and the insoluble solids were removed by filtration. The filtrate was washed with saturated sodium carbonate solution, dried with anhydrous sodium sulfate, concentrat...
Embodiment 3
[0067] A kind of synthetic method of 5-chloroisoindolinone, concrete steps are as follows:
[0068] Step 1: Esterification to obtain methyl 4-chloro-2-methylbenzoate
[0069] 30.0 g of 4-chloro-2-methylbenzoic acid, 2.0 ml of sulfuric acid and 300 ml of methanol were heated to reflux overnight. After distilling off the solvent, dilute with 300 ml of ethyl acetate, wash with 1 M, 100 ml*2 sodium hydroxide solution, dry over anhydrous sodium sulfate and concentrate to obtain 31.0 g of a colorless oily product with a yield of 93%.
[0070] Step 2: Dibromination reaction to give methyl 4-chloro-2-dibromomethylbenzoate
[0071] 31g of 4-chloro-2-dibromomethylbenzoic acid methyl ester, 60.0g NBS, 4.0g benzoyl peroxide and 300ml carbon tetrachloride obtained in step 1 were heated to reflux for 6 hours, filtered to remove insoluble solids, and the filtrate After washing with saturated sodium carbonate solution, it was dried with anhydrous sodium sulfate, concentrated and recrystalli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com