Tranexamic acid and preparation method thereof
A technology of tranexamic acid and p-toluic acid, which is applied in the direction of carboxylate preparation, cyanide reaction preparation, chemical instruments and methods, etc., can solve the problems of high raw material prices, unfavorable market promotion, and tranexamic acid yield Low-level problems, to achieve high yield and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of tranexamic acid is made by following steps:
[0037] Step S1: Add magnesium powder, iodine powder, and tetrahydrofuran into the reaction kettle, and use nitrogen for protection. Under the conditions of rotation speed of 100r / min and temperature of 155°C, p-bromotoluene is added dropwise, and reflux reaction is carried out for 1h. Introduce carbon dioxide until the reactor is full, and react for 30 minutes at a temperature of 3° C., add hydrochloric acid solution, and react for 1 hour to obtain p-toluic acid;
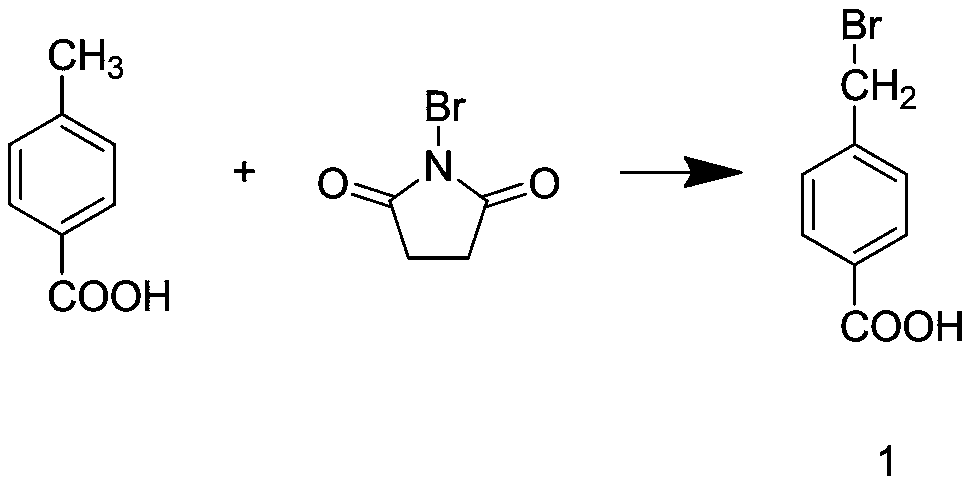
[0038] Step S2: Add the p-toluic acid, N-bromosuccinimide, and carbon tetrachloride prepared in step S1 into the reaction kettle, and stir until the toluic acid is After completely dissolving with N-bromosuccinimide, add dibenzoyl peroxide, and carry out a reflux reaction at a temperature of 80°C for 2 hours to obtain intermediate 1;
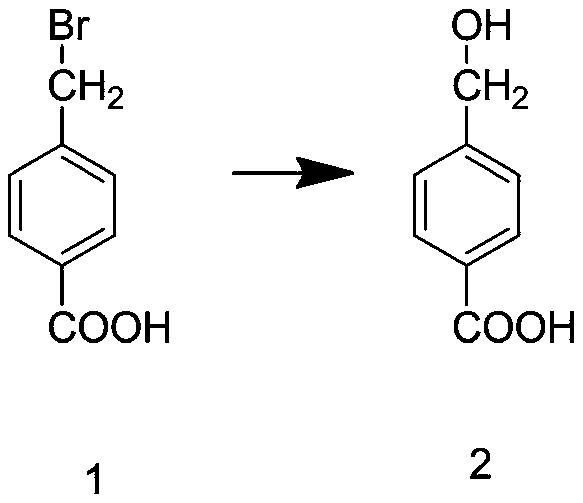
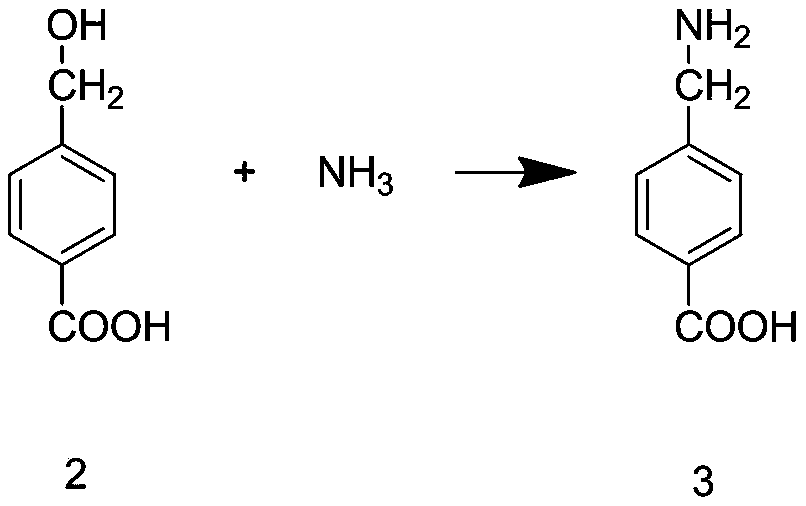
[0039] Step S3: Add sodium carbonate and deionized water into the reaction kettle, and stir at a speed of 200r / min unti...
Embodiment 2
[0043] A kind of tranexamic acid is made by following steps:
[0044] Step S1: Add magnesium powder, iodine powder, and tetrahydrofuran into the reaction kettle, and use nitrogen to protect it. Under the conditions of rotation speed of 150r / min and temperature of 158°C, p-bromotoluene is added dropwise, and reflux reaction is carried out for 1.5h , feed carbon dioxide to fill the reactor, and react at a temperature of 4°C for 35 minutes, add hydrochloric acid solution, and react for 1.3 hours to obtain p-toluic acid;
[0045]Step S2: Add the p-toluic acid, N-bromosuccinimide, and carbon tetrachloride prepared in step S1 into the reaction kettle, and stir until the toluic acid is After completely dissolving with N-bromosuccinimide, add dibenzoyl peroxide, and carry out a reflux reaction at a temperature of 85°C for 3 hours to obtain intermediate 1;
[0046] Step S3: Add sodium carbonate and deionized water into the reaction kettle, and stir at a speed of 250r / min until the sod...
Embodiment 3
[0050] A kind of tranexamic acid is made by following steps:
[0051] Step S1: Add magnesium powder, iodine powder, and tetrahydrofuran into the reaction kettle, and protect it with nitrogen gas. Under the conditions of rotation speed of 200r / min and temperature of 160°C, p-bromotoluene is added dropwise, and reflux reaction is carried out for 2 hours. Introduce carbon dioxide until the reactor is full, and react for 40 minutes at a temperature of 5°C, add hydrochloric acid solution, and react for 1.5 hours to obtain p-toluic acid;
[0052] Step S2: Add the p-toluic acid, N-bromosuccinimide, and carbon tetrachloride prepared in step S1 into the reaction kettle, and stir until the toluic acid is After completely dissolving with N-bromosuccinimide, add dibenzoyl peroxide, and carry out a reflux reaction at a temperature of 90°C for 4 hours to obtain intermediate 1;
[0053] Step S3: Add sodium carbonate and deionized water into the reaction kettle, and stir at a speed of 300r / m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com