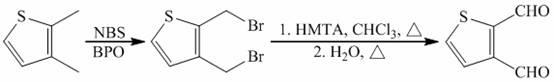Preparation method of 2, 3-thiophenedicarboxaldehyde
A technology of dialdehyde-based thiophene and dimethyl thiophene, which is applied in organic chemistry and other fields, and achieves the effects of easy-to-obtain raw materials, low production costs, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 2,3-Dimethylthiophene (11.22 g, 0.10 mol), benzoyl peroxide (0.24 g, 1.0 mmol), dissolved in 80 mL of carbon tetrachloride, vigorously stirred and heated to reflux, slowly Add a mixture of N-bromosuccinimide (35.60 g, 0.2 mol) and benzoyl peroxide (0.24 g, 1.0 mmol). After the addition is complete, continue the reaction for 3 h, cool to room temperature, and remove the formed butyl Diimide, add water to wash the filtrate, wash the organic layer with sodium bicarbonate solution, sodium chloride solution, dry with anhydrous potassium carbonate, evaporate the solvent by rotary evaporator, and purify the crude product by vacuum distillation to obtain the solid product 2, 20.3 g of 3-bis(bromomethyl)thiophene (yield 76%). Melting point: 49~50℃, 1 H NMR (solvent CDCl3), δ: 5.50 (2H, s), 5.29 (2H, s), 3.00 (1H, d, J=6.0 Hz), 2.72 (1H, d, J=6.0 Hz).
[0018] Hexamethylenetetramine (29.44 g, 0.21 mol) was dissolved in 100 mL of chloroform, and 2,3-bis(bromomethyl)thiophene (27...
Embodiment 2
[0020] 2,3-Dimethylthiophene (22.44 g, 0.20 mol), benzoyl peroxide (0.72 g, 3.0 mmol), dissolved in 160 mL of chloroform, stirred vigorously and heated to reflux, then slowly added in batches A mixture of N-bromosuccinimide (71.20 g, 0.4 mol) and benzoyl peroxide (0.72 g, 3.0 mmol), after the addition was complete, continue to react for 3 h, then cool to room temperature, and filter to remove the formed butanediol imide, adding water to wash the filtrate, the organic layer was washed with sodium bicarbonate solution and sodium chloride solution, dried with anhydrous potassium carbonate, the solvent was evaporated by a rotary evaporator, and the crude product was purified by vacuum distillation to obtain a solid product 2,3 - Bis(bromomethyl)thiophene 42.7 g (79% yield).
[0021] Hexamethylenetetramine (61.69 g, 0.44 mol) was dissolved in 200 mL of chloroform, and 2,3-bis(bromomethyl)thiophene (54 g, 0.2 mol) was slowly added dropwise under vigorous stirring. Heat spontaneousl...
Embodiment 3
[0023] 2,3-Dimethylthiophene (11.22 g, 0.10 mol), benzoyl peroxide (0.36 g, 1.5 mmol), dissolved in 80 mL of carbon tetrachloride, vigorously stirred and heated to reflux, slowly Add a mixture of N-bromosuccinimide (35.60 g, 0.2 mol) and benzoyl peroxide (0.36 g, 1.5 mmol). After the addition is complete, continue the reaction for 3 h, cool to room temperature, and remove the formed butyl Diimide, add water to wash the filtrate, wash the organic layer with sodium bicarbonate solution, sodium chloride solution, dry with anhydrous potassium carbonate, evaporate the solvent by rotary evaporator, and purify the crude product by vacuum distillation to obtain the solid product 2, 3-bis(bromomethyl)thiophene 19.2 g (yield 71%).
[0024] Hexamethylenetetramine (32.66 g, 0.23 mol) was dissolved in 100 mL of chloroform, and 2,3-bis(bromomethyl)thiophene (27 g, 0.1 mol) was slowly added dropwise under vigorous stirring. Heat spontaneously to reflux, heat to reflux for 30 min when the re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com