Metal organic framework based on imidazole sulfonic acid as well as preparation method and application
A metal organic framework, imidazole sulfonic acid technology, applied in organic chemistry, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve problems such as poor catalytic effect, and achieve less catalyst dosage , The effect of obvious catalytic effect and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1: the preparation of organic ligand L
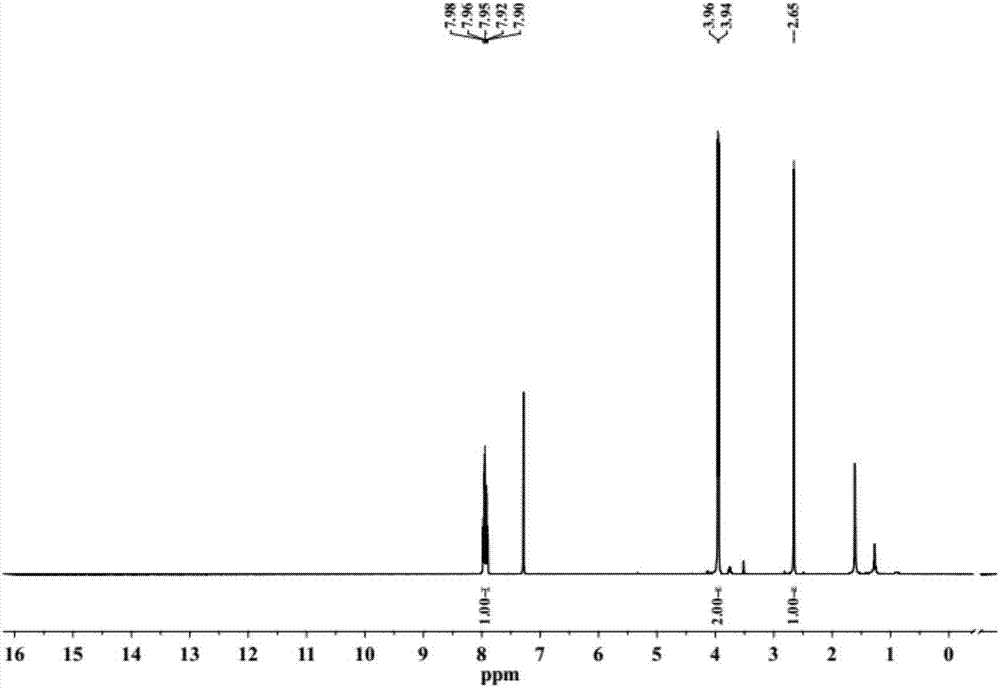
[0102] (1) 2-Methyl-terephthalic acid (0.9g, 5mmol) was mixed with methanol (50mL), reacted under the catalysis of concentrated sulfuric acid (5mL), and refluxed for 12 hours. Cooling, suction filtration to obtain the crude product is a white solid, which is intermediate A, and the productive rate is 95.0%. 1 H-NMR spectrum see figure 1 .
[0103](2) In a 100mL round bottom flask, add 2-methyl-terephthalic acid dimethyl ester (1.04g, 5mmol), bromosuccinimide (1.335g, 7.5mmol), AIBN (0.246g, 1.5 mmol), and then added benzene (45 mL), heated to 80° C., and refluxed for 12 hours. After cooling, the solvent was evaporated under reduced pressure to obtain a crude product. Dissolve in dichloromethane, filter to obtain the filtrate, remove the solvent under reduced pressure, and separate by column chromatography (petroleum ether:dichloromethane=1:1) to obtain 1.45 g of light yellow semi-solid, which is intermediate B, wit...
Embodiment 2
[0106] Example 2: Reactivity of Ligands with Sulfonic Acid Compounds
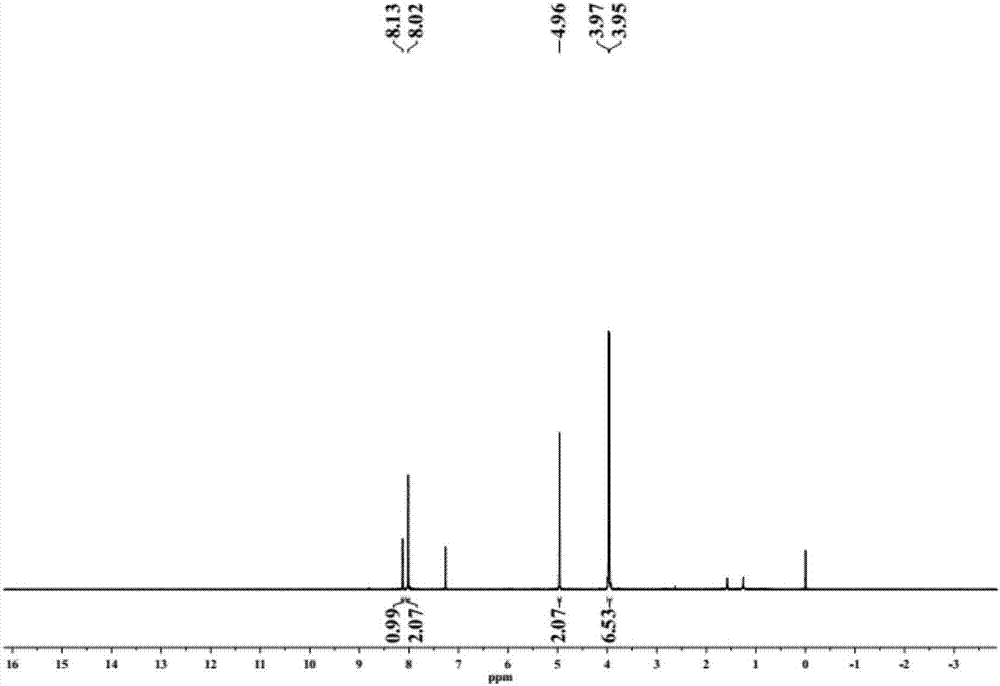
[0107] Intermediate C (0.274g, 1mmol) obtained in Example 1 and propyl group sultone (0.112g, 1mmol) in equimolar amounts were refluxed in 30mL acetone for 5h, and the solvent was removed by rotary evaporation to obtain product D. 1 H-NMR spectrum see Figure 5 .
Embodiment 3
[0108] Example 3: Preparation of metal-organic frameworks based on imidazole ligands
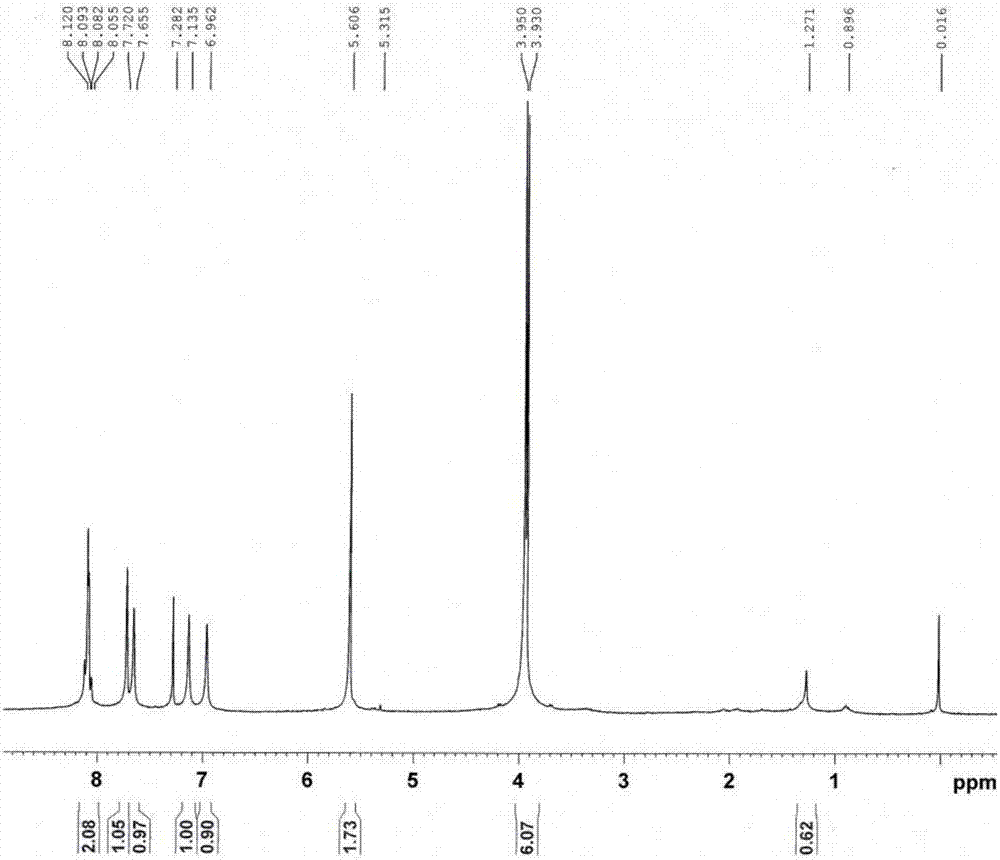
[0109] ZrCl 4 (28.8 mg, 0.12 mmol) and glacial acetic acid (0.342 mL), dissolved in 4.8 mL of DMF by sonication (20 min). Then organic ligand L (29.52 mg, 0.12 mmol) was added into the autoclave, and the solution was sonicated for 10-30 min. Then the mixture was heated to 120°C for 24-48h. Cool to room temperature, then centrifuge to obtain a white crystalline powder, wash with fresh DMF (~10-20ml×3), organic solvent (~10-20ml×3), centrifuge, and then in 40~80℃ Vacuum drying to obtain powder metal organic framework crystalline material, SEM photo see Figure 6 , see the PXRD spectrum Figure 7 , the crystals after digestion 1 H-NMR spectrum see Figure 8 .
[0110] Wherein, "~10-20 ml×3" means about 10-20 ml was washed 3 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com