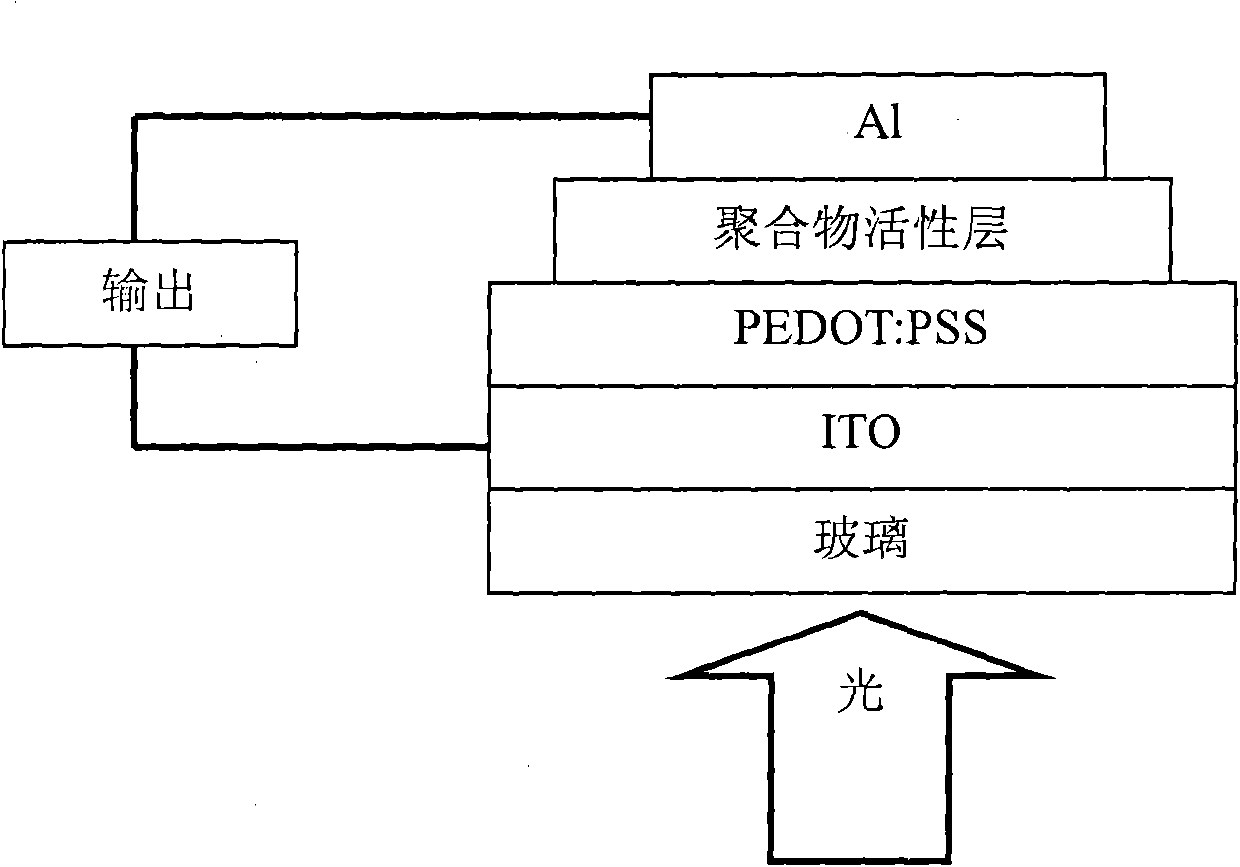
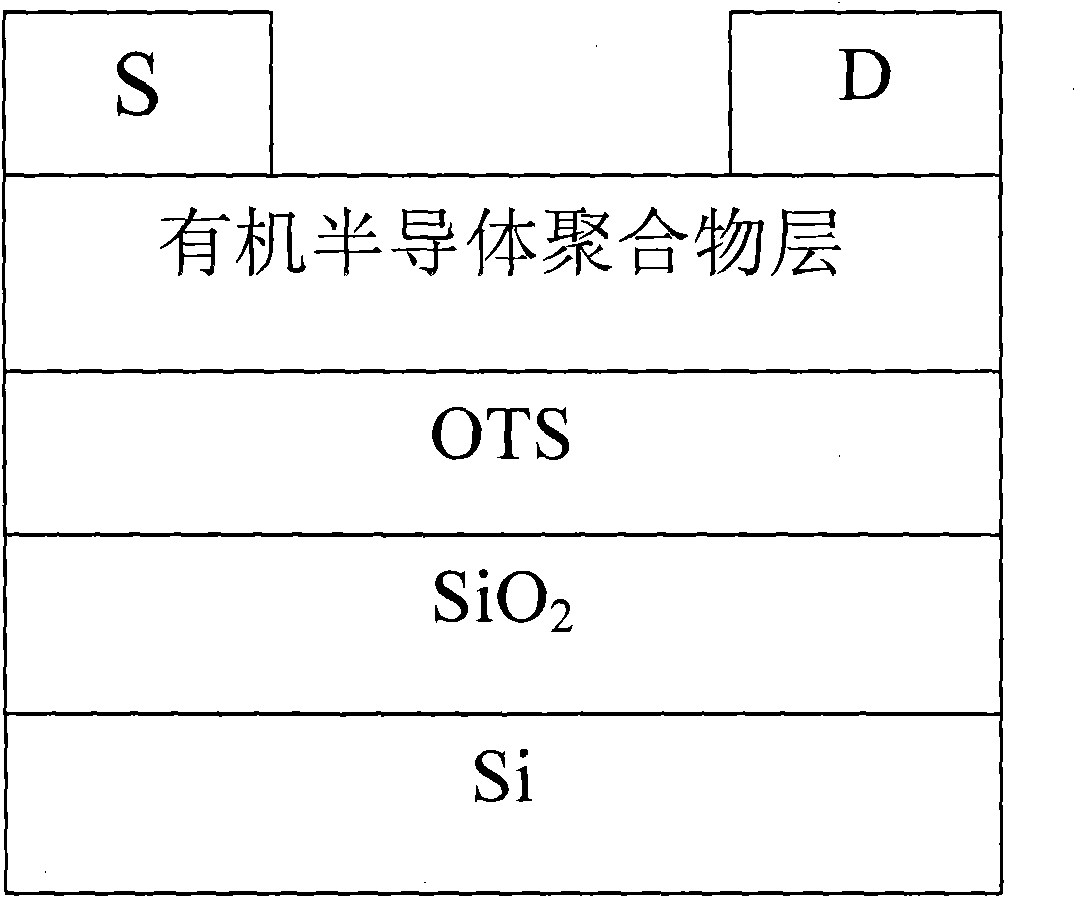
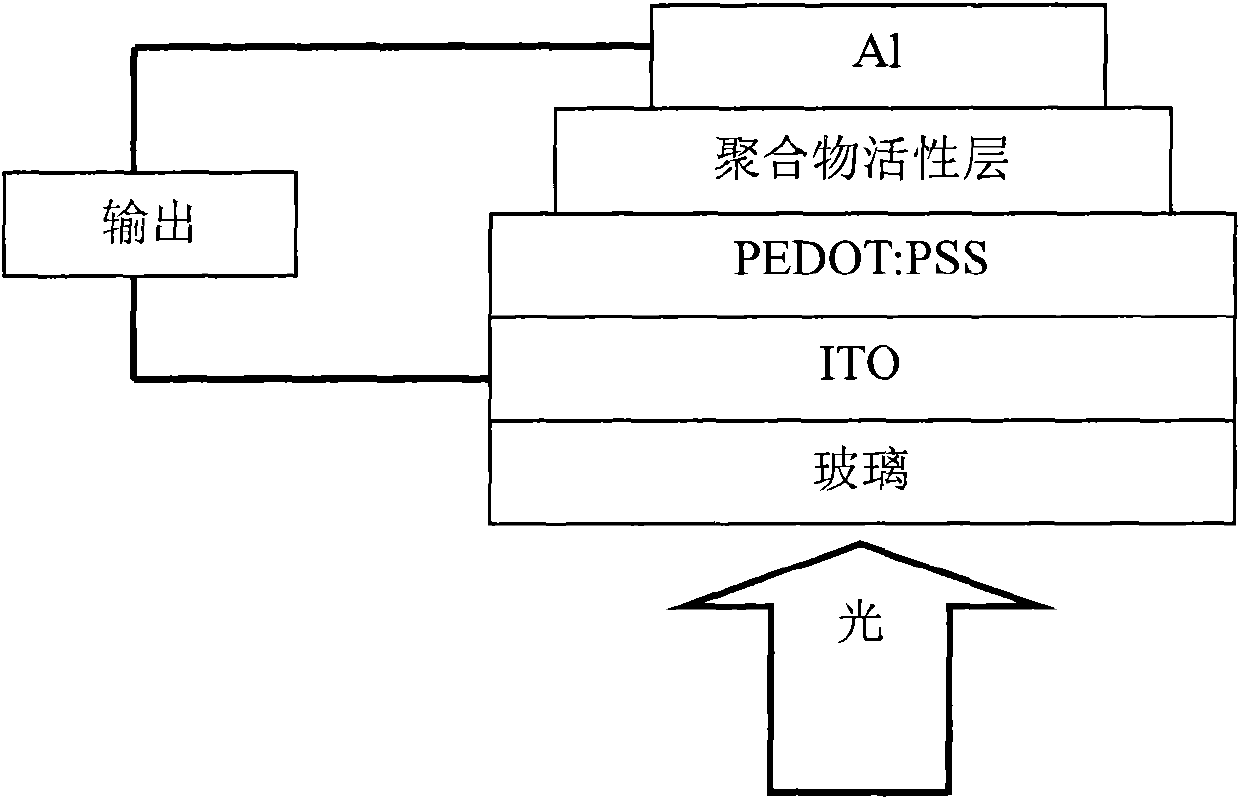
Perylene diimide-fluorene-thiophene and (3, 4-b) pyrazine conjugated polymer and preparation method and application thereof
A technology of perylene tetracarboxylic acid diimide and conjugated polymer is applied in the field of organic compound synthesis, and can solve the problems such as the collection efficiency of the carrier electrode that is not effectively utilized, the conversion efficiency is low, and the carrier mobility is low. , to achieve the effects of easy operation and control, improved utilization, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of N,N'-two-(3,4,5-tri-dodecyloxybenzene)-1,7-dibromo-3,4,9,10-perylenediimide:
[0051]
[0052] The specific synthetic steps are:
[0053] In the reaction flask, add 0.148g 1,7-dibromo-3,4,9,10-perylene tetraanhydride, 0.54g 3,4,5-tri-dodecyloxy-1-aminobenzene and 12ml propane acid, ultrasonically oscillated for 10 minutes to mix the reactants thoroughly, and then passed nitrogen gas into the reactants for 30 minutes, then stopped feeding nitrogen gas, heated the reactants to 80°C and reacted for 48 hours, cooled to room temperature after the reaction was completed, and added three Methane chloride was dissolved, and the organic layer was washed with sodium bicarbonate solution to obtain a red suspension, which was filtered, and anhydrous magnesium sulfate was added to the filtrate to dry to remove water, and finally spin dry. The product was obtained after column separation (dichloromethane:petroleum ether=3:1). MS (EI) m / z: 1806 (M+).
Embodiment 2
[0055] Preparation of 2,3-diphenyl-5.7-bis(2-thiophene)thieno[3,4-b]pyrazine:
[0056]
[0057] Dissolve 0.2mmol of 3',4'-diamino-2,2':5',2"-terthiophene and 0.28mmol of diphenylethanedione in 10ml of methanol, and react at 60°C for 1 hour to obtain A mixture containing the target reaction product. The mixture was subjected to column chromatography after removing the solvent under reduced pressure, and then recrystallized for purification. MS (EI) m / z: 453 (M+).
Embodiment 3
[0059] Preparation of 5,7-bis(5-bromothiophene)-2,3-diphenylthieno[3,4-b]pyrazine:
[0060]
[0061] Dissolve 150 mg of 2,3-diphenyl-5.7-bis(2-thiophene)thieno[3,4-b]pyrazine and 2.5 times NBS in 10 ml of THF, and react at room temperature for 28 hours to obtain the target reaction product. The solvent was removed from the reaction product under reduced pressure, and the obtained product was recrystallized in DMF. MS (EI) m / z: 610 (M+).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com