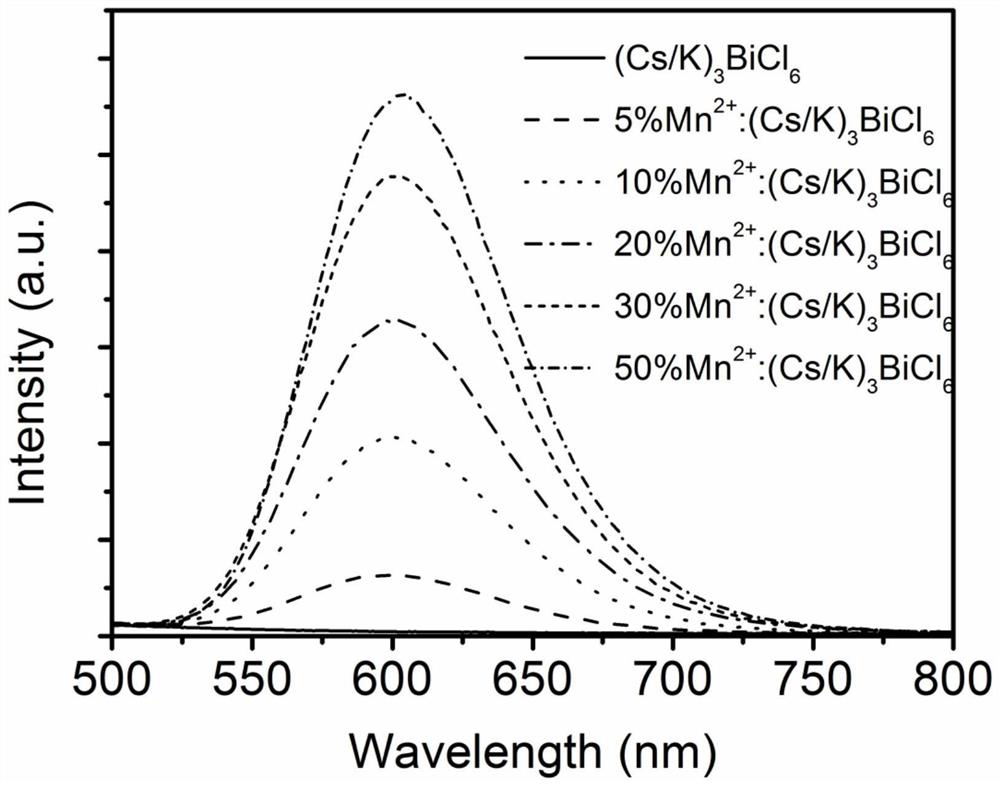
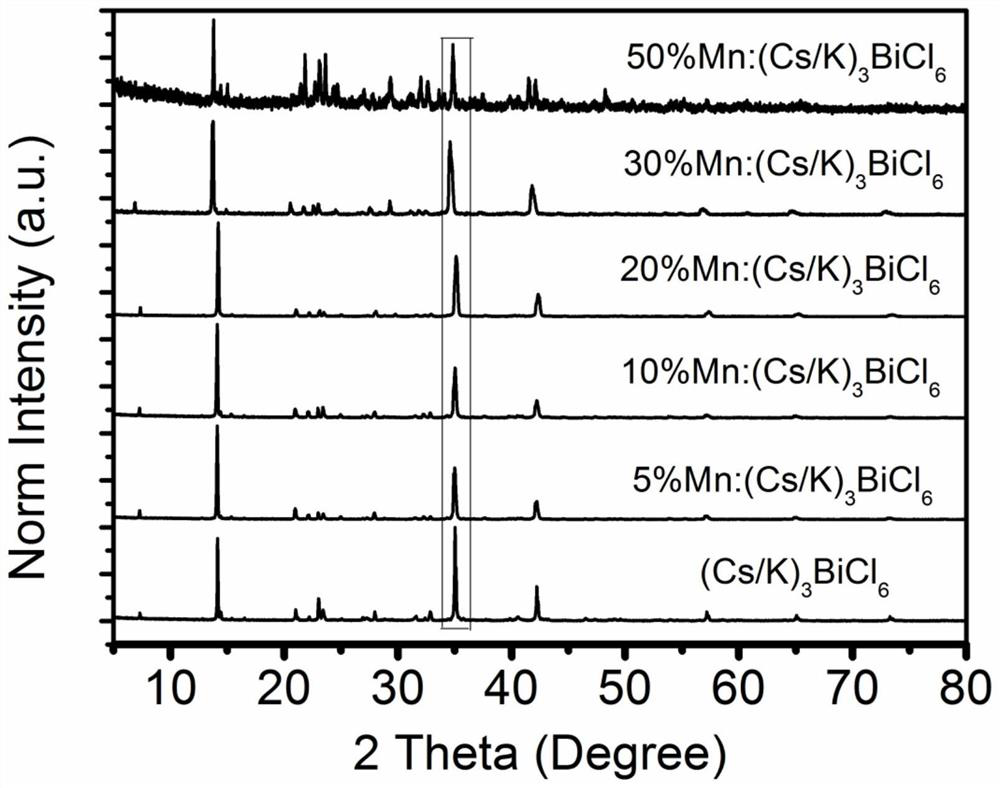
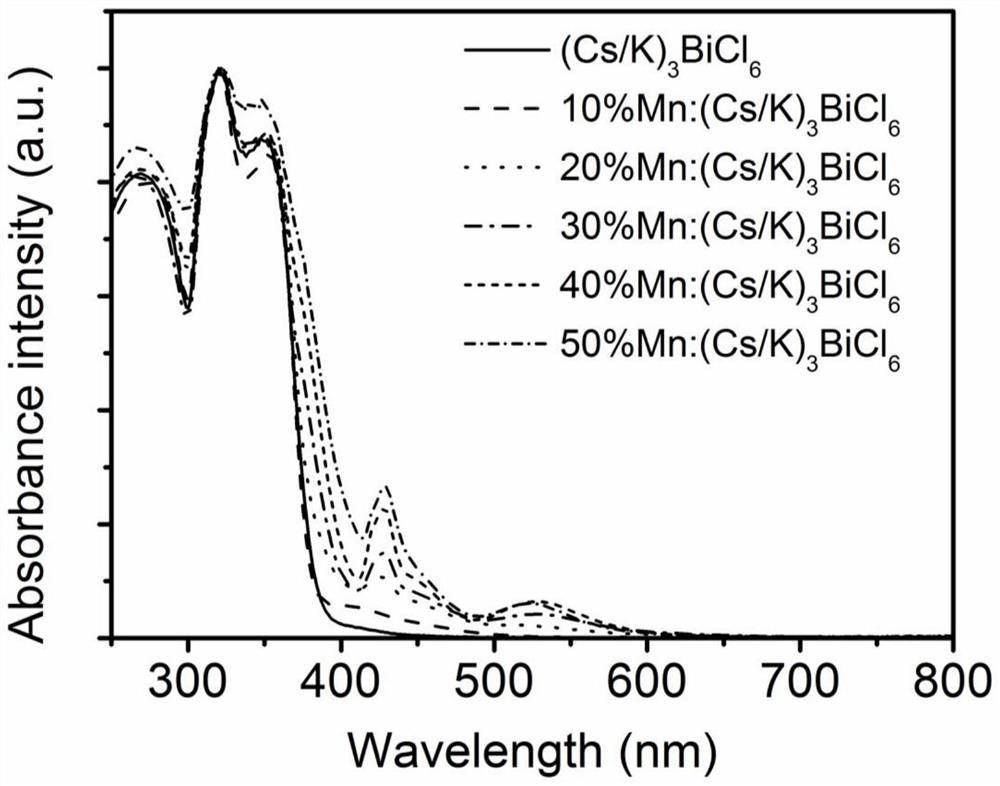
Mn-doped (Cs/K)3BiCl6 perovskite derivative material as well as preparation method and application thereof
A derivative and perovskite technology, applied in the field of photoluminescence fluorescence of perovskite derivatives, can solve the problems of poor photoelectric performance of lead-free halide perovskite, achieve high photoluminescence efficiency, wide application prospects, easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Place 1mmol of cesium chloride, 0.5mmol of potassium chloride and 0.5mmol of bismuth chloride in a polytetrafluoroethylene lining, and add 5mL of concentrated hydrochloric acid;
[0028] (2) Put the polytetrafluoroethylene lining of step (1) into the reaction kettle and seal it, move it to an oven and keep it at 180°C for 4 hours, and then slowly cool it down to room temperature;
[0029] (3) The reactant obtained in the step (2) was washed with ethanol for 3 times, placed in an oven, heated at 70° C. for 4 hours, and dried to obtain a solid powder.
Embodiment 2
[0031] (1) Place 1 mmol of cesium chloride, 0.5 mmol of potassium chloride, 0.5 mmol of bismuth chloride and 0.025 mmol of manganese chloride in a polytetrafluoroethylene liner, and add 5 mL of concentrated hydrochloric acid.
[0032] (2) Put the polytetrafluoroethylene liner in step (1) into the reactor and seal it, then move it to an oven and keep it at 180°C for 4 hours, then slowly cool it down to room temperature.
[0033] (3) Wash the reactant obtained in step (2) with ethanol for 3 times, place in an oven and heat at 70°C for 4 hours to dry to obtain Mn-doped (Cs / K) 3 BiCl 6 Perovskite derivative solid powder.
Embodiment 3
[0035] (1) Place 1 mmol of cesium chloride, 0.5 mmol of potassium chloride, 0.5 mmol of bismuth chloride and 0.05 mmol of manganese chloride in a polytetrafluoroethylene liner, and add 5 mL of concentrated hydrochloric acid.
[0036] (2) Put the polytetrafluoroethylene liner in step (1) into the reactor and seal it, then move it to an oven and keep it at 180°C for 4 hours, then slowly cool it down to room temperature.
[0037] (3) Wash the reactant obtained in step (2) with ethanol for 3 times, place in an oven and heat at 70°C for 4 hours to dry to obtain Mn-doped (Cs / K) 3 BiCl 6 Perovskite derivative solid powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com