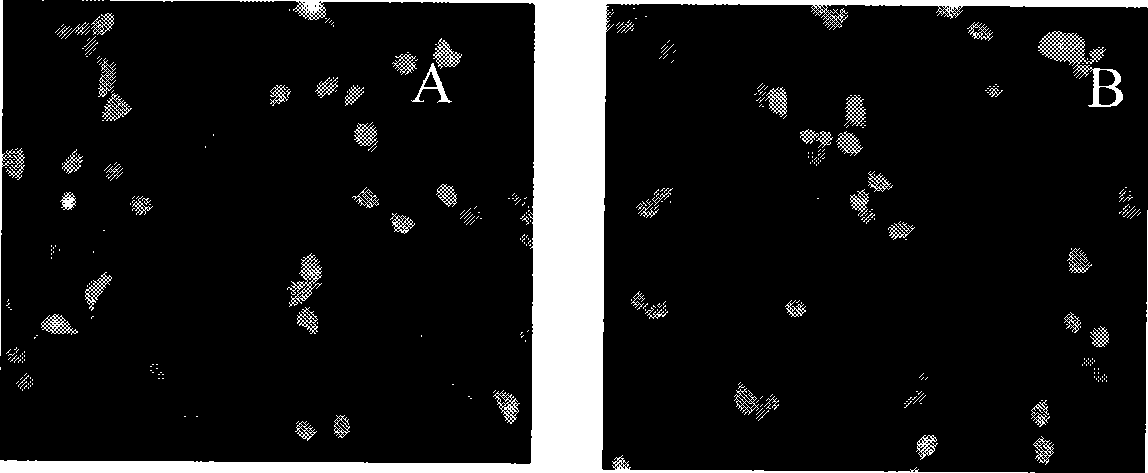
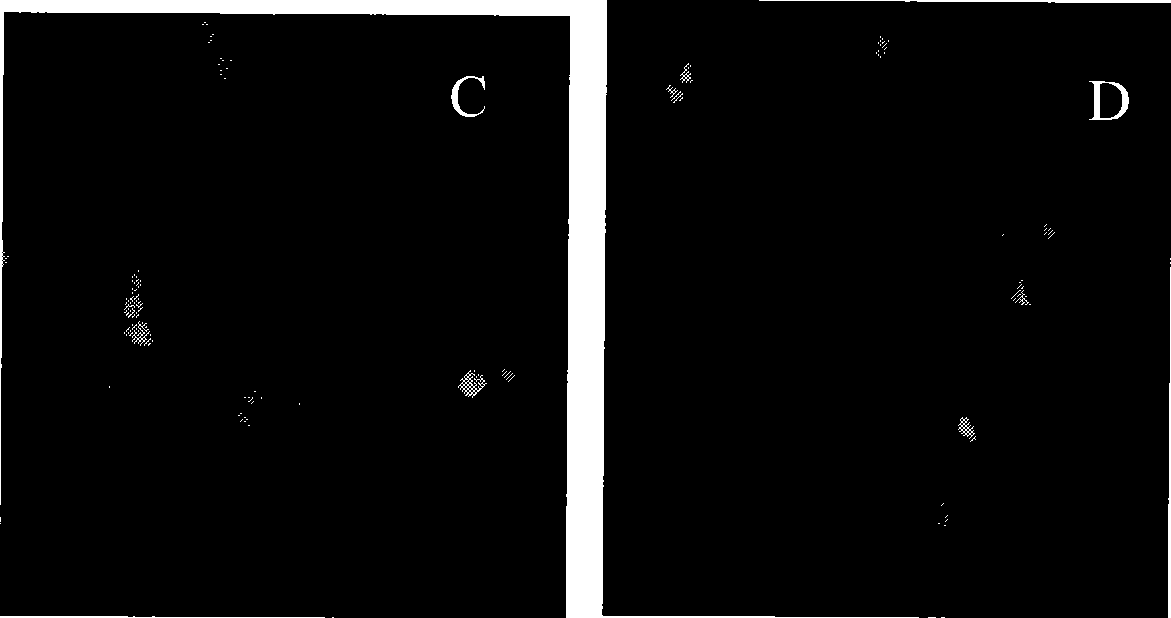
Polymethacrylate, preparation method and application thereof
A methacrylate and dimethylamino technology, applied in the application field of polymethacrylate in gene therapy, can solve the problem of unfavorable transport in vivo, damage to cell membrane structure, cell transfection and no research on terminal thiol oligomers. Cytotoxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0087] Example 1: Synthesis of 1,3,5-tris(2-(thiobenzenesulfonyl-2-propyl))benzene
[0088] In a 500ml round bottom flask, add 100ml carbon tetrachloride, 0.1mol1,3,5-triisopropylbenzene, 0.45mol N-bromosuccinimide (NBS), 20mg benzoyl peroxide catalyst, reflux After 48 hours, filter, wash with saturated sodium bicarbonate three times, dry over anhydrous magnesium sulfate, and distill off the solvent to obtain a yellow oily liquid with a yield of 85%. Add the yellow oily liquid to 100ml of chloroform solvent, add 0.45mol of thiobenzenesulfonyl magnesium bromide, reflux for 48h, separate by column chromatography, and distill off the solvent to obtain 1,3,5-tri(2-(thiobenzene Sulfonyl-2-propyl))benzene.
example 2
[0089] Example 2: Synthesis of 1,3,5-tris(2-(xanthogenyl-2-propyl))benzene
[0090] Add 100ml carbon tetrachloride, 0.1mol1,3,5-triisopropylbenzene, 0.45mol N-bromosuccinimide, 20mg benzoyl peroxide catalyst into a 500ml round bottom flask, reflux for 48h, filter, Wash with saturated sodium bicarbonate, dry over anhydrous magnesium sulfate, and distill off the solvent to obtain a yellow oily liquid with a yield of 80%. Add the yellow oily liquid to 100ml of chloroform solvent, add sodium ethyl xanthate, reflux for 48h, separate by column chromatography, and distill off the solvent to obtain 1,3,5-tris(2-(ethyl xanthate-2 -Propyl))benzene.
example 3
[0091] Example 3: Synthesis of TEX-PDMAEMA
[0092] In a 5ml round bottom flask, add DMAEMA (1g), azobisisobutyronitrile (4mg), 1,3,5-tris(2-(thiobenzenesulfonyl-2-propyl))benzene (80mg) and 2ml of tetrahydrofuran, fully deoxygenated, vacuum-sealed, and placed in a water bath at 60°C for 48 hours. The 1,3,5-terminal trisulfonate polymer—TEX-PDMAEMA was obtained, and excess hexane was added to precipitate and isolate the product. for polymer 1 H NMR characterization, D 4 -Methanol and D-chloroform as solvents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com