O-carborane derivative, synthesis method and application thereof
A technology of o-carborane and synthesis method, which is applied in the field of o-carborane derivatives to achieve the effects of increasing cell affinity and increasing polarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
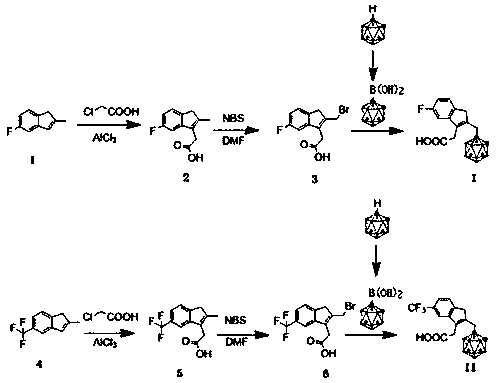
Embodiment 1~3
[0033] Embodiment 1~3 is the preparation embodiment of compound 1, compound 2
Embodiment 1
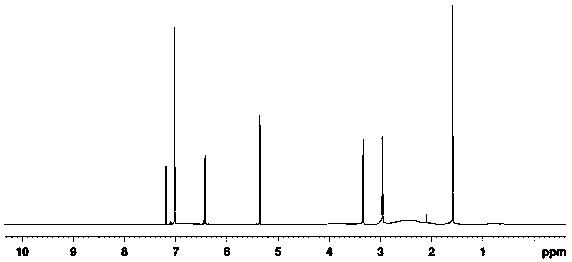
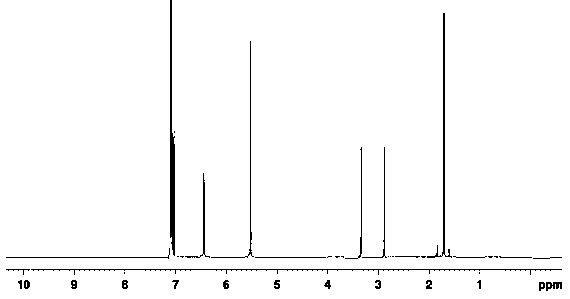
[0035] 1.48g of compound 1 (5-fluoro-2-methyl-1H-indene), 20ml of dichloromethane and 1.4g of aluminum trichloride were added to a 100ml single-necked flask, and the temperature of the system was reduced to 0-10 ℃, add 1.0g of 1-chloroacetic acid dropwise, the temperature rises to 15-20℃ and reacts for 4h. MgSO 4 After drying, the mother liquor was filtered and distilled under reduced pressure to obtain 1.9 g of compound 2, with a yield of 95%. The detection parameters are:
[0036] 1 H-NMR: 1.78 (s,3H), 2.93 (t, J = 5.5 Hz, 2H), 3.24 (t, J = 5.4 Hz, 2H), 5.37 (s, 1H), 6.67–6.68 (d, J = 8.5 Hz, 1H), 7.02 (s, 1H), 7.36–7.38 (d, J =8.7 Hz, 1H).
Embodiment 2
[0038] Roughly the same as Example 1, except that dichloromethane was replaced by chloroform to obtain 2.0 g of compound 2 with a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com