Preparation method of phloroglucinol
A technology for phloroglucinol and resorcinol, which is applied in the field of chemical material preparation, can solve problems such as difficulty in extraction, and achieve the effects of shortening reaction time, reducing production cost and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
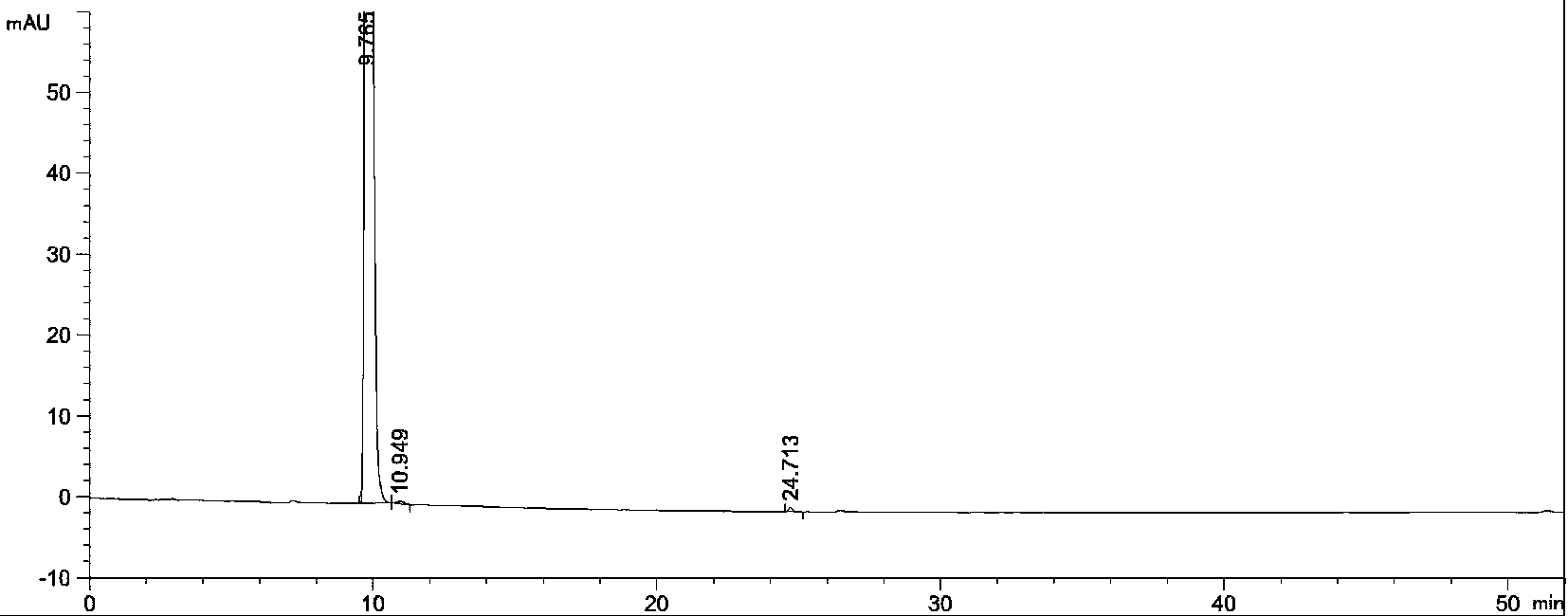
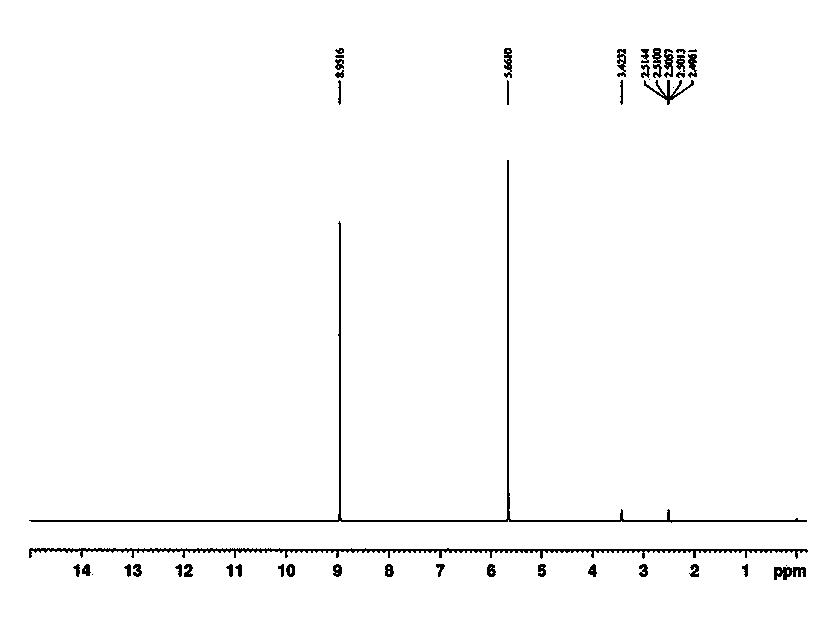
Image
Examples
Embodiment 1
[0032] The preparation method of phloroglucinol of the present invention, the detailed steps of described preparation method are as follows:
[0033] a, bromination reaction: with resorcinol as raw material, first resorcinol is added in the reaction vessel, then chloroform is added for dissolving, after dissolving, N-bromosuccinimide is dissolved in chloroform to obtain N -The bromosuccinimide solution was added dropwise to the reaction container, and the N-bromosuccinimide solution was added dropwise for 30 minutes. After the dropwise addition, the temperature was raised to 60°C, and the reaction was carried out at this temperature for 1 hour. Finally, the reaction liquid was obtained, and the obtained reaction liquid was distilled out of chloroform at normal pressure, and the obtained liquid was distilled under reduced pressure, and the 150-160 ° C fraction was collected under a vacuum degree of 0.098 MPa, and the obtained fraction was cooled to normal temperature to obtain 4...
Embodiment 2
[0040] The preparation method of phloroglucinol of the present invention, the detailed steps of described preparation method are as follows:
[0041] a. Bromination reaction: using resorcinol as raw material, first add resorcinol into the reaction vessel, then add dichloromethane to dissolve, and dissolve N-bromosuccinimide in dichloromethane The N-bromosuccinimide solution obtained in is added dropwise to the reaction vessel, and the time for adding the N-bromosuccinimide solution is 35 minutes. After the dropwise addition, the temperature is raised to 40°C. Reaction for 3 hours, after the reaction, the reaction solution was obtained, and dichloromethane was distilled from the obtained reaction solution under normal pressure, and the obtained liquid was distilled under reduced pressure, and the fraction at 150-160°C was collected under a vacuum degree of 0.098MPa, and the obtained fraction was distilled Cool to normal temperature to obtain 4-bromoresorcinol;
[0042] The rat...
Embodiment 3
[0048] The preparation method of phloroglucinol of the present invention, the detailed steps of described preparation method are as follows:
[0049] a, bromination reaction: with resorcinol as raw material, first resorcinol is added in the reaction vessel, then acetone is added to dissolve, after dissolution, N-bromosuccinimide is dissolved in acetone to obtain N -The bromosuccinimide solution was added dropwise to the reaction container, and the N-bromosuccinimide solution was added dropwise for 40 minutes. After the dropwise addition, the temperature was raised to 80°C, and the reaction was carried out at this temperature for 0.5h. After the reaction, the reaction liquid was obtained, and the obtained reaction liquid was distilled to remove acetone under normal pressure. After the acetone was distilled off, the obtained liquid was distilled under reduced pressure, and the 150-160 ° C fraction was collected under a vacuum of 0.098 MPa, and the obtained fraction was cooled to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com