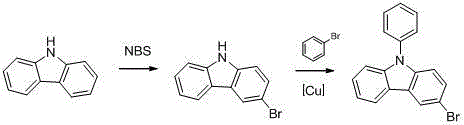
Preparation method of N-phenyl-3-bromocarbazole
A technology of bromocarbazole and phenyl, which is applied in the field of organic compound synthesis and achieves the effects of convenient industrial production, high yield and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A kind of preparation method of N-phenyl-3-bromocarbazole, comprises following two steps:
[0026] The preparation of the first step 3-bromocarbazole:
[0027] Add 50.14 g (0.30 mol) of carbazole to a 500 mL four-neck flask equipped with magnetic stirring and a thermometer, add 200 mL of ethyl acetate and stir at room temperature to dissolve, slowly add 100 mL of ethyl acetate to dissolve 53.43 g (0.30mol) N-bromosuccinimide (NBS), react for 2 to 4 hours after dropping, control in TLC until the reaction is complete, filter, wash with 40% aqueous sodium hydroxide solution, separate liquid, keep the organic layer, evaporate The organic solvent was removed to obtain 69.42 g of solid, the crude product yield was 94.25%, recrystallized from ethanol, filtered and dried to obtain 52.04 g of 3-bromocarbazole, and the recrystallized yield was 74.96%.
[0028] The preparation of the second step N-phenyl-3-bromocarbazole:
[0029] Add 52.04g (0.21mol) of 3-bromocarbazole, 32.76g...
Embodiment 2
[0031] A kind of preparation method of N-phenyl-3-bromocarbazole, comprises following two steps:
[0032] The preparation of the first step 3-bromocarbazole:
[0033] Add 49.94 g (0.30 mol) of carbazole to a 500 mL four-neck flask equipped with a magnetic stirrer and a thermometer, add 200 mL of ethyl acetate and stir at room temperature to dissolve, lower the temperature to 0°C and slowly add 100 mL of ethyl acetate to dissolve while stirring 69.38g (0.39mol) of NBS, reacted for 2 to 4 hours after dropping, controlled by TLC until the end of the reaction, filtered, washed with 40% aqueous sodium hydroxide solution, separated, kept the organic layer, and evaporated the organic solvent to obtain 71.38g of solid , the yield of crude product was 96.73%, recrystallized from ethanol, filtered and dried to obtain 57.78g of 3-bromocarbazole, and the yield of recrystallized was 80.96%.
[0034] The preparation of the second step N-phenyl-3-bromocarbazole:
[0035] Add 57.78g (0.235m...
Embodiment 3
[0037] A kind of preparation method of N-phenyl-3-bromocarbazole, comprises following two steps:
[0038] The preparation of the first step 3-bromocarbazole:
[0039] Add 50.04 g (0.30 mol) of carbazole to a 500 mL four-neck flask equipped with a magnetic stirrer and a thermometer, add 200 mL of ethyl acetate and stir at room temperature to dissolve, lower the temperature to 0°C and slowly add 100 mL of ethyl acetate to dissolve while stirring 47.94 g (0.30mol) of bromine, reacted for 2 to 4 hours after dropping, controlled in TLC until the end of the reaction, filtered, washed with 40% aqueous sodium hydroxide solution, separated, kept the organic layer, and evaporated the organic solvent to obtain a solid 68.92 g, the crude product yield was 94.05%, recrystallized from ethanol, filtered and dried to obtain 54.04 g of 3-bromocarbazole, and the recrystallized yield was 77.06%.
[0040] The preparation of the second step N-phenyl-3-bromocarbazole:
[0041] Add 54.04 g (0.22 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com