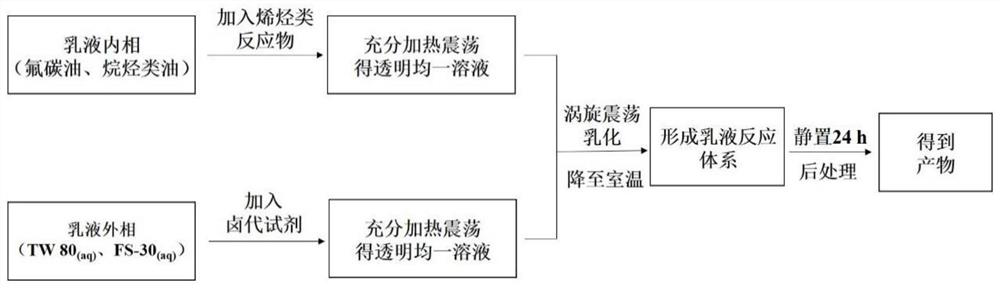
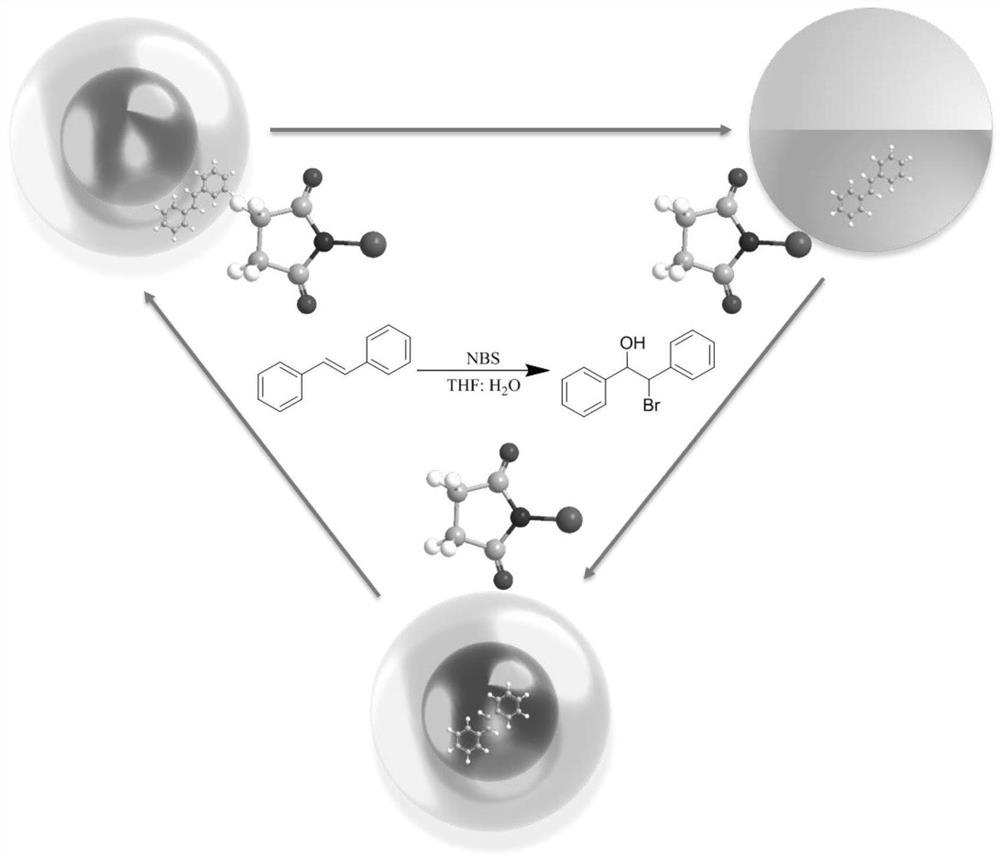
Method for synthesizing beta-brominated alcohol by anisotropic emulsion microreactor
An anisotropic, micro-reactor technology, applied in the field of chemistry, can solve problems such as limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
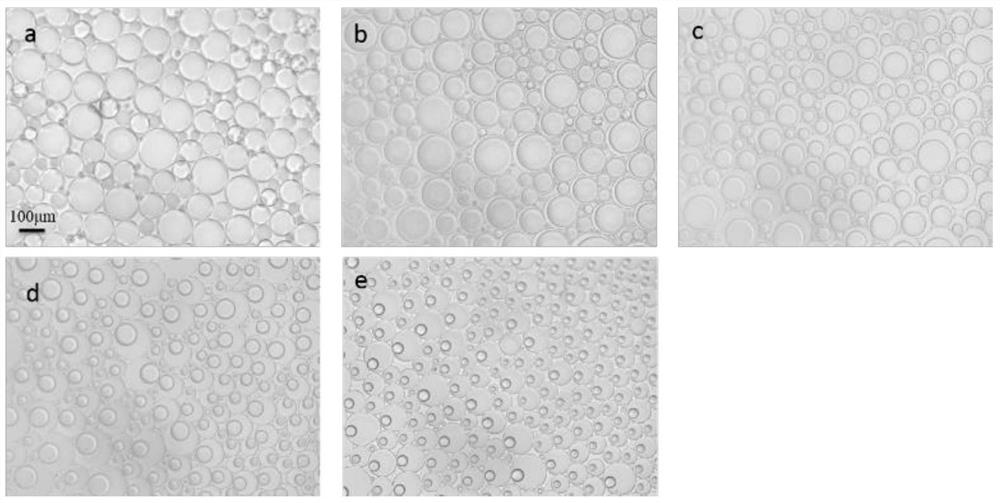
[0027] The volume ratio of oil to water is 1:1, and the volume ratio of FC-770 / n-heptane is changed to a-4:1; b-2:1; c-1:1; d-1:2; e-1:4 . Shake for 3 min under this condition to prepare the emulsion.
[0028] Authentication: from image 3 It can be seen that the volume ratio of FC-770 / n-heptane can form an emulsion under the conditions of 4:1; 2:1; 1:1; 1:2; 1:4, but the emulsion formed under the condition of the volume ratio of 4:1 is One-sided emulsion, while other volume ratios are anisotropic emulsions. With the decrease of the volume of FC-770 in the initial oil phase and the increase of the volume of n-heptane phase, the volume of FC-770 domains in the droplet also decreases gradually, and the volume of n-heptane domains gradually increases. The volume ratio of FC-770 / n-heptane domains can be varied from 4:1 to 1:4.
Embodiment 2
[0031] Keep 2wt% Tween 80 (aq) : n-heptane: FC-770 volume ratio is 2:1:1. Shake for 3 min under this condition to prepare the emulsion. Micrographs of droplets were taken under online temperature control at different temperatures. Sudan I dyes dissolve selectively in the n-heptane phase.
[0032] Authentication: from Figure 4 It can be seen that the temperature has an effect on the type of droplets. As the temperature increases to 45℃, the type of droplets changes from anisotropic emulsion to single-sided emulsion, and the anisotropy of the emulsion disappears.
Embodiment 3
[0035] Keep the aqueous phase:n-heptane:FC-770 volume ratio at 2:1:1. Wherein the surfactant aqueous solution is respectively a-2wt% Tween 80, b-1wt% Tween 80+0.31wt% FS-30, c-0.6wt% Tween 80+0.43wt% FS-30, d-0.62wt% FS- 30. Fluorescent dyes are selectively dissolved in the n-heptane phase. Shake for 3 min under this condition to prepare the emulsion.
[0036] Authentication: from Figure 5 It can be seen that the type of surfactant can affect the topology of the emulsion. With the decrease of Tween 80 and the increase of FS-30 in the water phase, the topology of the emulsion changes from F / H / W to H / F / W.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com