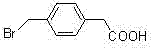
P-bromomethyl phenylacetic acid preparation method
A technology of bromomethylphenylacetic acid and methylphenylacetic acid, which is applied in the field of preparation of fine chemical intermediates and p-bromomethylphenylacetic acid, can solve the problems of difficult scale-up production and low yield, and achieve high yield and convenient The effect of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
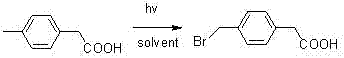
[0019] Put the raw material p-tolueneacetic acid (24 grams) in 250 ml of three ports, add 80 ml of chlorobenzene, add 30 grams of NBS, 0.6 grams of azobisisobutyronitrile, and heat to 85 ° C under mechanical stirring, the reaction starts Afterwards, the temperature will be automatically raised to 110°C, the reaction will be controlled at 90 to 110°C, and the reaction will be kept in this temperature range for 4 hours, monitored by TLC plate, and the reaction is complete. After cooling, a solid was precipitated. After filtration, the filter cake was washed with water and dried as much as possible by suction. Then the obtained white solid weighed 33.0 g after drying, the yield was 90%, and the HPLC purity was 98.7%.
[0020] 1 HNMR (DMSO-d6,300 MHz) δ: 12.2(s,1H),7.18-7.23(m,4H),4.69(s,2H),3.57(s,2H).
Embodiment 2
[0022] Put the raw material p-tolueneacetic acid (24 grams) in 250 ml of three mouths, add 90 ml of dichlorobenzene, add 30 grams of NBS, 0.6 grams of azobisisobutyronitrile, and heat at 85 ° C under mechanical stirring, and react After initiation, the temperature will be automatically raised to 115°C, the reaction will be controlled at 90 to 115°C, and the reaction will be kept in this temperature range for 5 hours, monitored by TLC plate, and the reaction is complete. After cooling, a solid was precipitated. After filtration, the filter cake was washed with water and dried as much as possible by suction. Then the obtained white solid weighed 32.3 g after drying, the yield was 88.1%, and the HPLC purity was 98.3%.
[0023] 1 HNMR (DMSO-d6,300 MHz) δ: 12.2(s,1H),7.18-7.23(m,4H),4.69(s,2H),3.57(s,2H).
Embodiment 3
[0025] Put the raw material p-tolueneacetic acid (24g) in a 250ml three-port, add 75ml of chlorobenzene, add 30g of NBS, 0.5g of benzoyl peroxide, heat to 85°C under mechanical stirring, After initiation under irradiation, the temperature will automatically rise to 110°C, the reaction will be controlled at 90 to 110°C, and the reaction will be kept in this temperature range for 8 hours, monitored by TLC plate, and the reaction is complete. After cooling, a solid was precipitated. After filtration, the filter cake was washed with water and dried as much as possible by suction. Then the obtained white solid weighed 33.7 g after drying, the yield was 91.9%, and the HPLC purity was 99.2%.
[0026] 1 HNMR (DMSO-d6,300 MHz) δ: 12.2(s,1H),7.18-7.23(m,4H),4.69(s,2H),3.57(s,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com