Preparation method of high-performance carboxyl-terminated low-molecular-weight fluorine-containing polymer
A low molecular weight, polymer technology, applied in the direction of additive processing, etc., can solve the problems of product thermal, chemical stability and functionalization, and achieve the effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
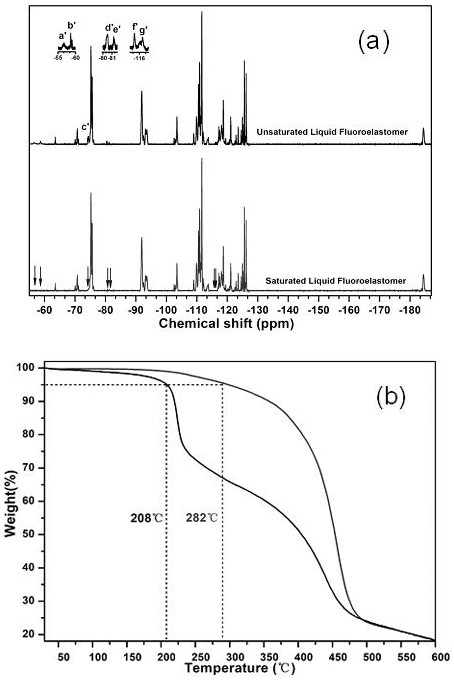
[0025] 10g of carboxyl-terminated low-molecular-weight fluoropolymer (carboxyl-terminated vinylidene fluoride-hexafluoropropylene binary copolymer, number average molecular weight 3500, double bond content of 1.47mol% or 0.17mmol / g, thermal decomposition temperature of 208°C, fluorine Content 64%), dissolved in 100ml tetrahydrofuran, placed in a 500ml single-necked flask. Add the fluorinating reagent N-fluoro-N'-(chloromethyl)triethylenediamine bis(tetrafluoroborate) (60.22mg, 0.17mmol) and the nucleophile silver fluoride (21.57mg, 0.17mmol) in sequence After 4 hours of reaction, the hydrogenation reagent lithium aluminum hydride (6.45mg, 0.17mmol) and the electrophile N-bromosuccinimide (30.26mg, 0.17mmol) were added, and the temperature was raised to 25°C for 4 hours of reaction. After the reaction, the organic phase was left to stand and collected, and the solvent was removed, and the product was vacuum-dried at 50-70° C. for 24 hours.
[0026] The product was subjected to...
Embodiment 2
[0029] Take 10g of carboxyl-terminated low-molecular-weight fluorine-containing polymer (carboxyl-terminated vinylidene fluoride-hexafluoropropylene binary copolymer, number average molecular weight 3200, double bond content of 1.15mol% or 0.12mmol / g, thermal decomposition temperature of 230°C, fluorine Content 63.8%), dissolved in 110ml acetone, placed in a 500ml single-necked flask. 1-Fluoro-4-methyl-1,4-diazabicyclo[2.2.2]octane tetrafluoroborate (57.57mg, 0.18mmol), potassium fluoride (10.46mg, 0.18mmol), After reacting for 4 hours, sodium borohydride (6.81mg, 0.18mmol) and N-fluorobenzenesulfonimide (56.76mg, 0.18mmol) were added, the temperature was raised to 30°C, and the reaction was carried out for 5 hours. After the reaction, the organic phase was left to stand and collected, and the solvent was removed, and the product was vacuum-dried at 50-70° C. for 24 hours.
[0030] product 19 According to F-NMR, the characteristic peak intensity corresponding to the double b...
Embodiment 3
[0033] 10g of carboxyl-terminated low-molecular-weight fluoropolymer (carboxyl-terminated vinylidene fluoride-hexafluoropropylene binary copolymer, number average molecular weight 2600, double bond content of 0.95mol% or 0.10mmol / g, thermal decomposition temperature 232°C, fluorine Content 62.7%), dissolved in 120ml of acetonitrile, placed in a 500ml single-necked flask. Add triethylamine hydrofluoride (32.24mg, 0.20mmol) and diethylaminosulfur trifluoride (24.18mg, 0.15mmol) successively. After 4 hours of reaction, add potassium borohydride (10.79mg, 0.20mmol), N-iodosuccinimide (33.75mg, 0.15mmol) was heated up to 35°C and reacted for 5 hours. After the reaction, the organic phase was left to stand and collected, and the solvent was removed, and the product was vacuum-dried at 50-70° C. for 24 hours.
[0034] product 19 According to F-NMR, the characteristic peak intensity corresponding to the double bond structure is obviously weakened, the calculated double bond content ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com