Ropivacaine nanometer lipid carrier temperature-sensitive in-situ gel and preparation method thereof
A nano-lipid carrier, ropivacaine technology, applied in the direction of capsule delivery, liquid delivery, microcapsules, etc., to achieve the effect of good reproducibility, not easy to lose, and easy to produce on a large scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation of ropivacaine nano-lipid carrier thermosensitive in situ gel
[0065] Dissolve 25 mg of ropivacaine (Jinan Dexinjia Biotechnology Co., Ltd.), 100 mg of stearic acid, 40 mg of oleic acid, and 100 mg of soybean lecithin (lipiod from Germany) in 5 ml of acetone, and heat to 75°C to completely dissolve to form an organic phase;
[0066] Dissolve 250mg of HS-15 (BASF, Germany) in 10ml of water for injection, and heat to 75°C to completely dissolve to form the water phase;
[0067] Inject the organic phase into the water phase at a temperature of 75°C and a stirring speed of 500 r / min, and stir for 2.5 hours to completely volatilize the organic phase to obtain colostrum;
[0068] Pour the colostrum into 25ml of water at 0-2°C, and stir in an ice bath at a speed of 500r / min for 2h to obtain a colloidal solution of ropivacaine nano-lipid carrier;
[0069] In an ice bath, slowly add the blank temperature-sensitive gel matrix (poloxamer 407 accounting for...
Embodiment 2
[0070] Example 2: Preparation of ropivacaine nano-lipid carrier thermosensitive in situ gel
[0071] Take 30mg of ropivacaine, 200mg of glyceryl monostearate, 40mg of medium-chain triglycerides (Tieling Beiya Pharmaceutical Oil Co., Ltd.) and 150mg of soybean lecithin dissolved in 5ml of ethanol, and heat to 80°C to dissolve completely, forming The organic phase;
[0072] Dissolve 200mg of HS-15 in 15ml of water for injection, heat to 80°C to dissolve completely, and form the water phase; at a temperature of 80°C and a stirring speed of 1000r / min, inject the organic phase into the water phase, stir for 3 hours, and make The organic phase is completely volatilized to obtain colostrum;
[0073] Pour colostrum into 20ml of water at 0-2°C, and stir in an ice bath at a speed of 1000r / min for 2 hours to obtain a colloidal solution of ropivacaine nano-lipid carrier;
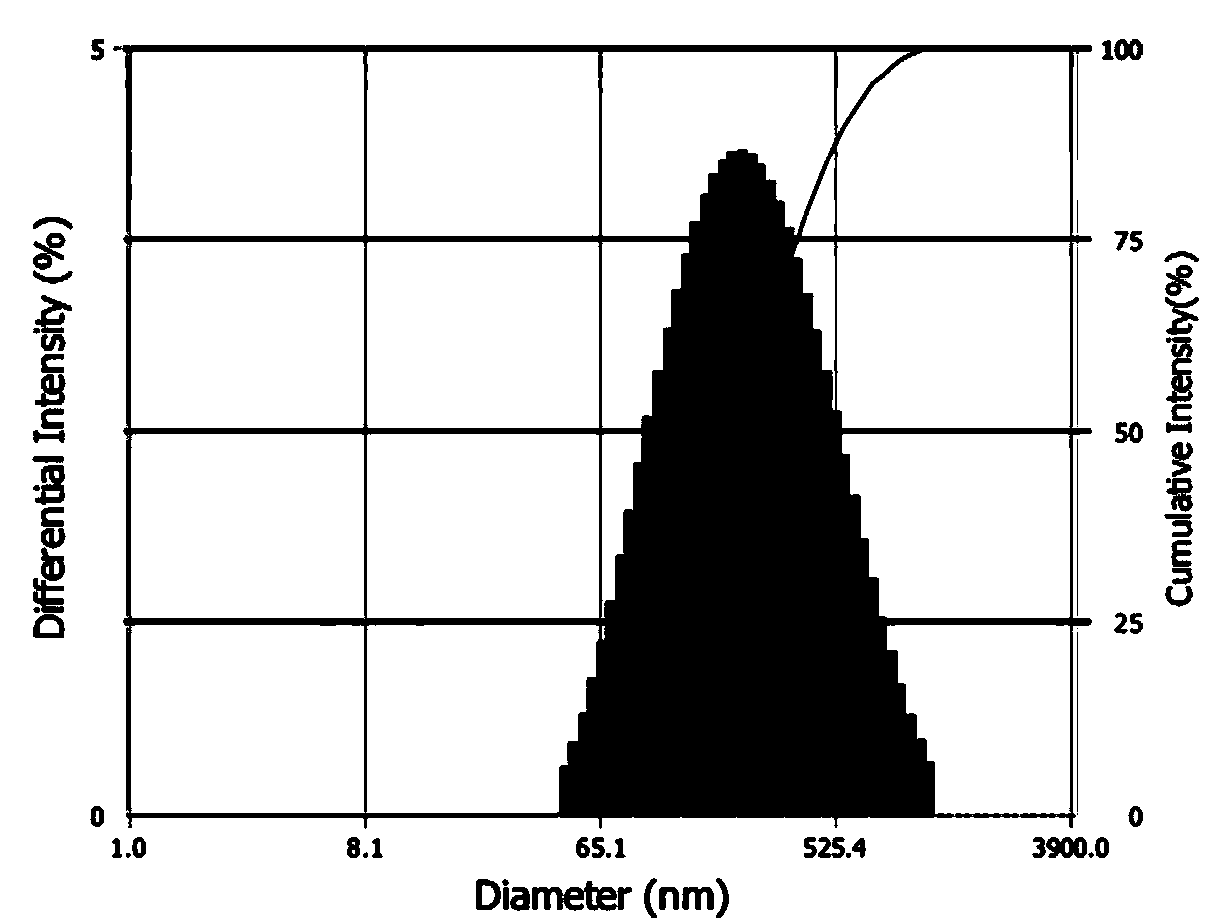
[0074] After diluting with water, observe its morphology with a H-7000 transmission electron microscope, as shown i...
Embodiment 3
[0076] Example 3: Preparation of ropivacaine nano-lipid carrier thermosensitive in situ gel
[0077] Dissolve 30mg of ropivacaine, 150mg of glyceryl monostearate, 50mg of oleic acid, and 150mg of lecithin (German lipiod) in 10ml of ethanol, and heat to 75°C to completely dissolve to form an organic phase;
[0078] Dissolve 300mg of sodium lauryl sulfate in 20ml of water for injection, heat to 75°C to dissolve completely, and form the water phase;
[0079] Inject the organic phase into the water phase at a temperature of 75°C and a stirring speed of 1000 r / min, and stir for 4 hours to completely volatilize the organic phase to obtain colostrum;
[0080] Pour the colostrum into 20ml of water at 0-2°C, and stir in an ice bath for 4 hours at a rotational speed of 1000r / min to obtain a colloidal solution of ropivacaine nano-lipid carrier;
[0081] In an ice bath, the blank thermosensitive gel matrix (poloxamer 407 accounting for 19% of the total system) was slowly added to the rop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com