Platinum-icodextrin-polycaprolactone macromolecular compound, nano drug delivery system and application of nano drug delivery system
A technology of icodextrin and polycaprolactone, applied in the direction of active ingredients of heavy metal compounds, preparations for in vivo tests, medical preparations of non-active ingredients, etc., can solve problems such as poor cycle stability and poor water solubility, and achieve Improved stability, small drug particle size, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
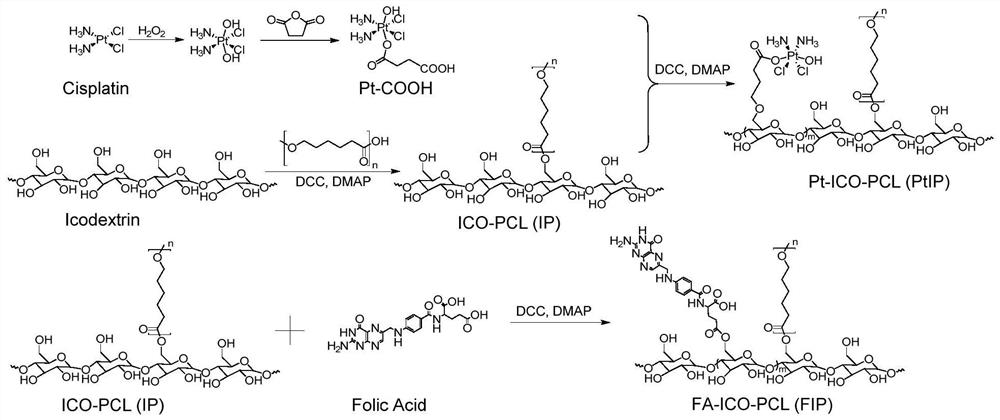
[0073] The present invention also provides a platinum - preparing polycaprolactone macromolecule compound, comprising the steps of - icodextrin:
[0074] S1, oxidizing cisplatin into tetvalent platinum compound with hydrogen peroxide, reacting with an acid anhydride, and purifying the compound PT-COOH after separation.
[0075] S2, the carboxyl group on the polycaprolactone is esterified with the hydroxyl group in the Ai Topylide, and the compound ICO-PCL is obtained after separating purification;
[0076] S3, the carboxyl group on the compound Pt-COOH of step S1 is esterified in the hydroxyl group in which the hydroxyl group in the compound ICO-PCL in step S2 is esterified, and the platinum-Ai Tour is obtained after separating and purification. Ester macromolecular compound.
[0077] In some embodiments, the step S1 is specifically: cisplatin reaction mixture and hydrogen peroxide, divalent platinum oxide cisplatin become tetravalent platinum, isolated and purified tetravalent pl...
Embodiment 1
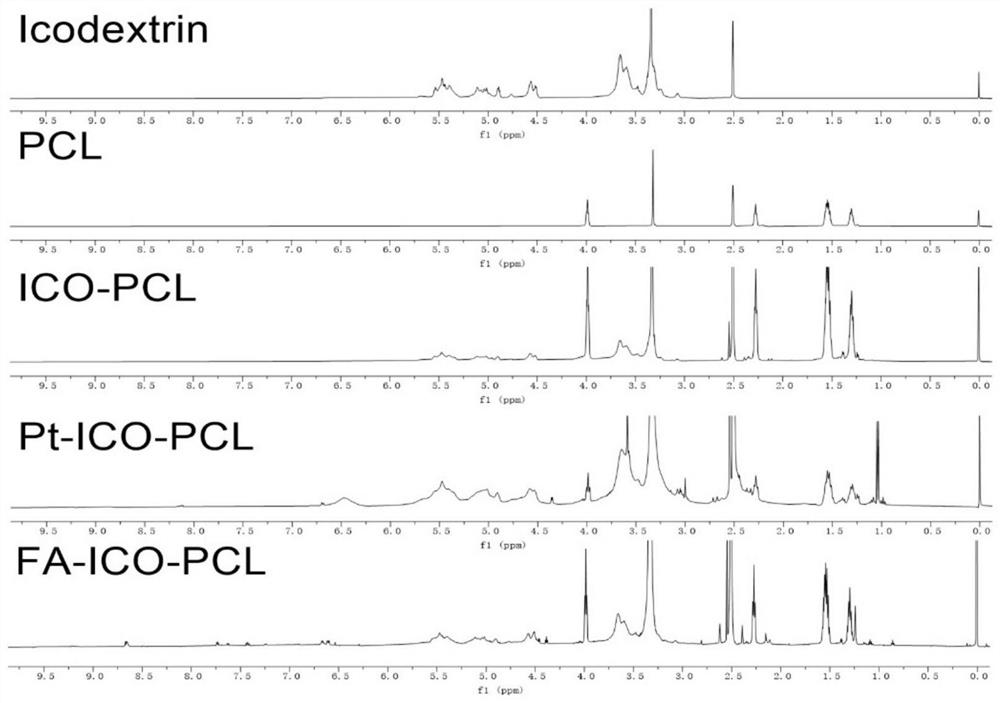
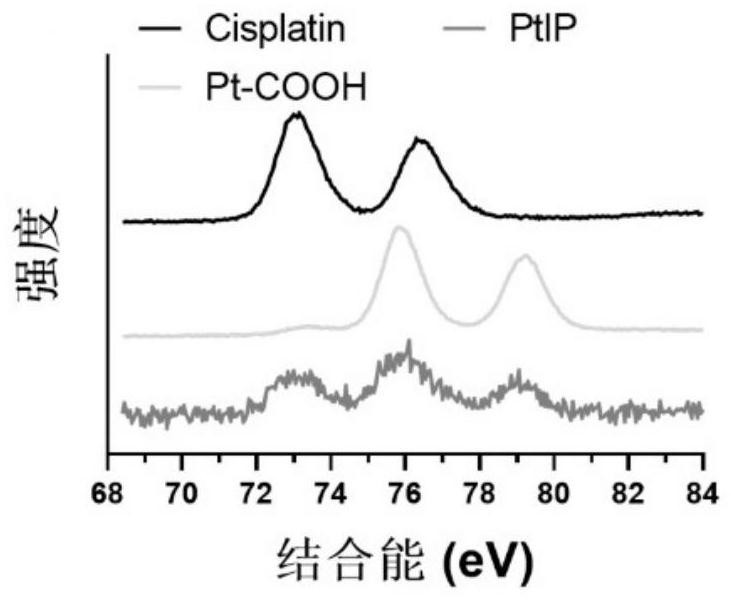
[0099] Example 1 Compound Preparation and verification and FIP embodiment PtIP
[0100] The embodiment according to the present embodiment figure 1 Preparation of compound represented by the flowchart PtIP, following these steps:
[0101] (1) Weigh 1g Cisplatin (Cisplatin, 3.33mmol) was placed in a 100mL round bottomed flask, and the flask was successively added 35mL 30% hydrogen peroxide (10-fold excess) and 25mL of ultrapure water. 50 ℃ reflux 1h. After completion of the reaction, the ice bath, the product was recrystallized precipitate, the supernatant was collected by centrifugation, and then washed with ethanol, washed three times with ether, and dried in vacuo to give a white solid powder is the oxidation product of cisplatin.
[0102] (2) Weigh accurately Previous cisplatin oxidation product 334mg (1mmol) and succinic anhydride 100mg (1mmol) was added to 50mL round bottom flask, followed by addition of molecular sieve-dried 30mL anhydrous dimethyl sulfoxide in a round botto...
Embodiment 2
[0112] Example 2 DiR embodiment entrapped preparation and characterization of nanoparticles based DPtFIP FIP co-assembled and PtIP
[0113] This embodiment utilizes several different dialysis prepared Pt-ICO-PCL and FA-ICO-PCL ratio nanoparticles, the specific operation is as follows:
[0114] (1) in accordance with the ratio of the Pt-ICO-PCL FA-ICO-PCL mass were 9: 1, 8: 2 and 7: 3 Weigh two samples, so that the total mass of the two samples was 5mg.
[0115] (2) to step (1) the ratio of each was added a solution of dimethylsulfoxide 3mL 3mgDiR dissolution, and then samples were applied to a molecular weight cutoff of the dialysis bag 3500Da. At room temperature, dialyzed in ultrapure water stirring 24h, 2h replaced once every fresh ultrapure water, the dialysis bag was collected after completion of the dialysis. A small amount of ultrafiltration (molecular weight cutoff 10 KDa) and concentrated to a final volume of 5 mL; most lyophilized stand.
[0116] By dynamic light scatter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com