Use of a mixture of modified glucose polymers for reducing tumor metastasis
A technology for tumor metastasis and composition, which can be used in anti-tumor drugs, active ingredients of hydrocarbon compounds, medical preparations containing active ingredients, etc., and can solve problems such as harmfulness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
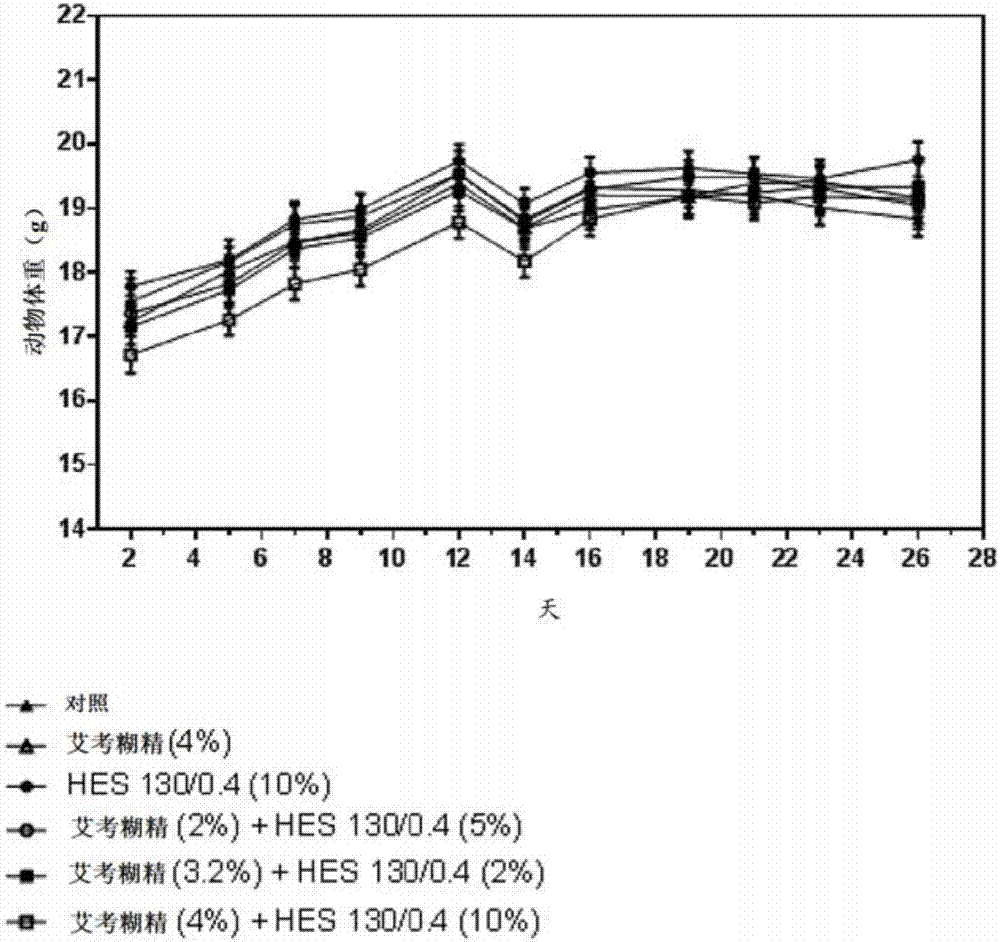
[0133] Overview: Adult female BALB / c nude mice were treated with human colon adenocarcinoma LS174T ( CL-188 TM ) after inoculation with saline, icodextrin (4%), alone or in combination with 4% icodextrin solution Blood volume surrogate (10%) and a single i.p. injection of a solution containing 10% HES 130 / 0.4 dissolved in 4% icodextrin solution were treated to determine tumor cell growth and body weight during the course of the experiment.
[0134] substance:
[0135] Saline (0.9% NaCl) (Lot No. 134002, B. Braun Melsungen AG, Melsungen, Germany) was used as a control. Test articles containing hydroxyethyl starch (HES) Blood volume substitute (10%) (poly(O-2-hydroxyethyl) starch (HES 130 / 0.4) 100 g / L, NaCl 9 g / L) (batch number 14FC3308) was purchased from Fresenius Kabi as a ready-to-use product Fresenius Kabi Deutschland GmbH (Bad Homburg, Germany). 4% icodextrin (40g / L, sodium chloride 5.4g / L, sodium lactate 4.5g / L, calcium chloride 257mg / L, magnesium chloride 61mg / L)...
Embodiment 2
[0152] Overview: Adult female BALB / c nude mice were treated with human colon adenocarcinoma LS174T ( CL-188 TM ) after inoculation, with saline, icodextrin (4%), icodextrin (7.5%) alone Single i.p. injections of 10%, or different combinations of HES130 / 0.4 and icodextrin were treated to determine tumor cell growth and body weight over the course of the experiment.
[0153] substance:
[0154] Saline (0.9% NaCl) (Lot No. 1440030, B. Braun Melsungen AG, Melsungen, Germany) was used as a control. Test articles containing hydroxyethyl starch (HES) 10% (poly(O-2-hydroxyethyl) starch (HES130 / 0.4) 100g / L, NaCl 9g / L) (batch number 14FC3308) was purchased from Fresenius Kabi Germany Co., Ltd. (Fresenius Kabi Deutschland) as a ready-to-use product GmbH) (Bad Homburg, Germany). 4% icodextrin (40g / L, sodium chloride 5.4g / L, sodium lactate 4.5g / L, calcium chloride 257mg / L, magnesium chloride 61mg / L) (batch number 13892004, Baxter AG, (Austria, Vienna)) and 7.5% icodextrin (75g / L, ...
Embodiment 3
[0168] Example 3: Overview
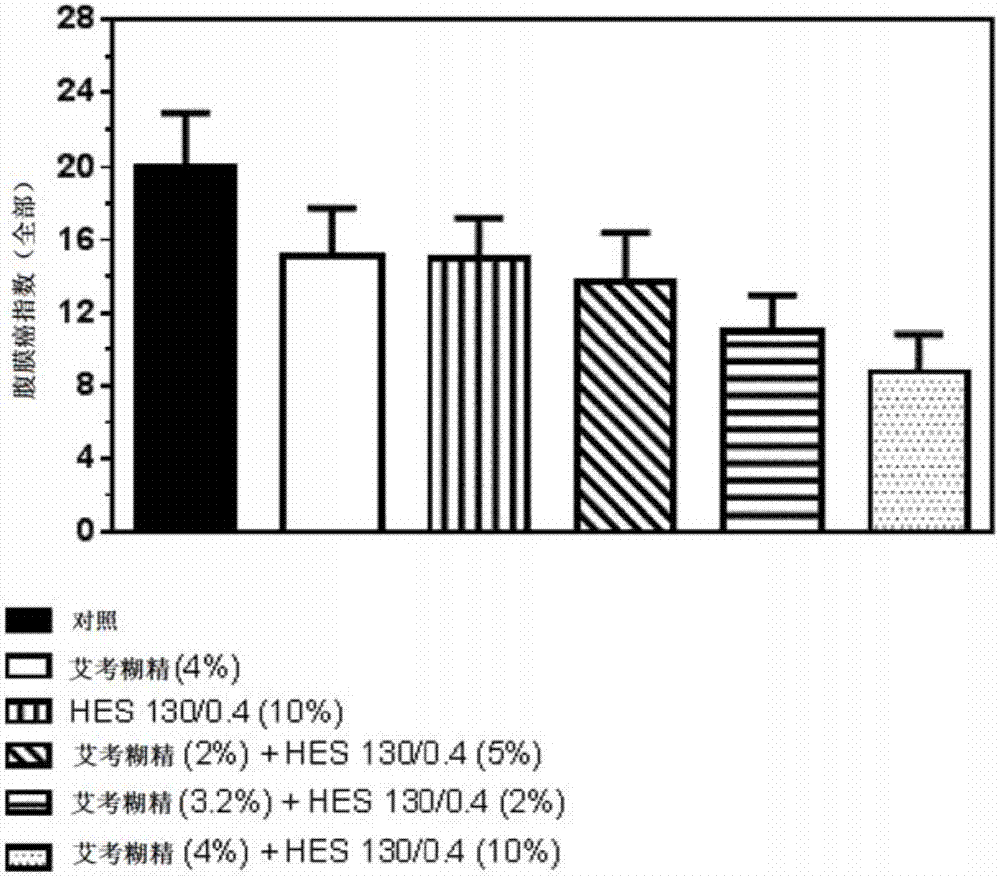
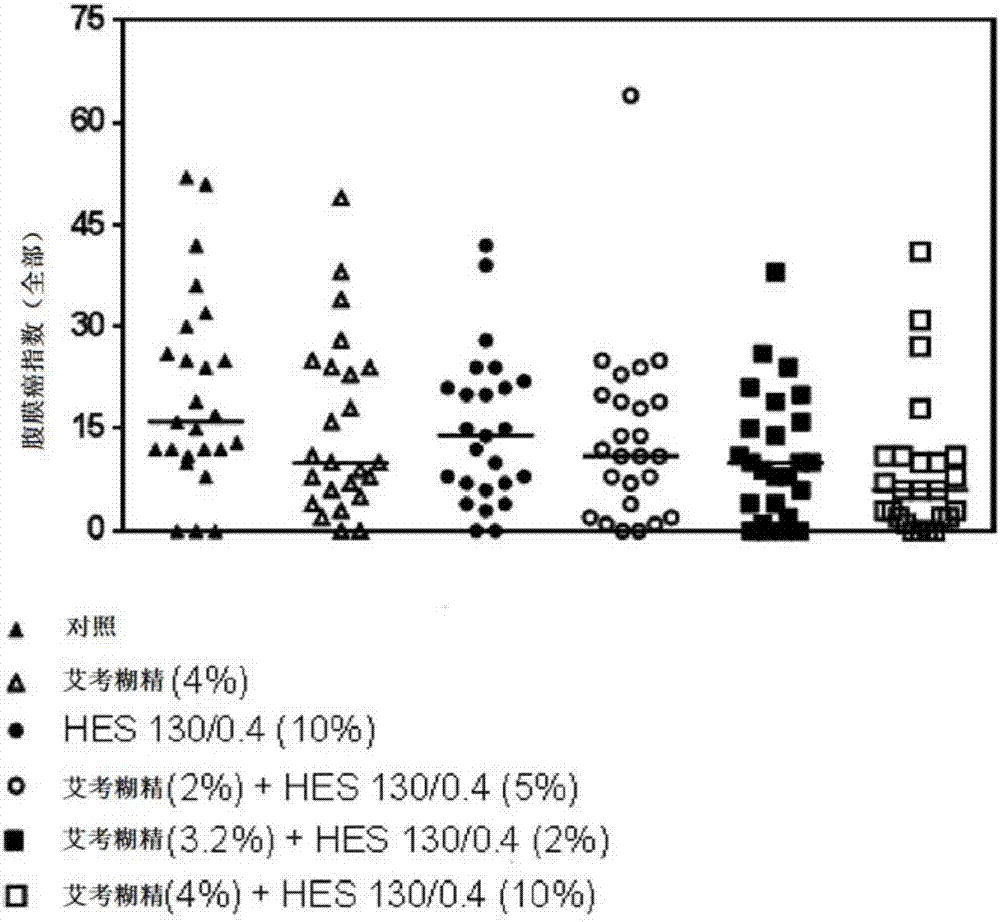
[0169] Figure 11 A compilation of the data obtained in Examples 1 and 2 is shown. To make the data comparable, the total PCI value for the control (saline) was set at 100% and other values were expressed as % of the control. As will be appreciated, solutions containing up to 5% icodextrin and up to 10% HES were shown to provide improved protection from tumor cell nesting compared to both the control and solutions containing a single polysaccharide. Further increasing the icodextrin concentration to above 7.5% and / or increasing the HES concentration to 20% resulted in no further improvement. Although the solution containing 7.5% icodextrin did not increase the protective effect compared to the 4% icodextrin solution, it was still effective compared to the administration of the control solution. Without wishing to be bound by theory, it can be concluded from these data that the protective effect may not be due to the osmotic (hypertonic) effect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com